Summary
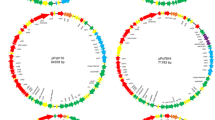
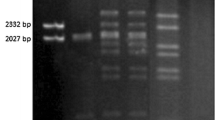
Pseudomonas syringae pv. phaseolicola strain LR719 contains a 150 kilobase pair (kb) plasmid pMC7105, stably integrated into its chromosome. Occasionally, single colony isolates of this strain contain an excision plasmid. Eight unique excision plasmids were selected and characterized by BamHI restriction endonuclease and blot hybridization analyses. These plasmids ranged in size from 35 to 270 kb; the largest contained approximately 130 kb of chromosomal DNA sequences. Restriction maps of pMC7105 were developed to deduce the site of integration and to identify the fragments in which recombination occurred to produce each excision plasmid. The eight excision plasmids were arranged into five classes based on the sites where excision occurs. A 20 kb region of pMC7105, which includes BamHI fragment 9 and portions of adjacent fragments, is present in all excision plasmids and thought to contain the origin of replication. The site of integration on pMC7105 maps within BamHI fragment 8. This fragment shows homology with seven other BamHI fragments of pMC7105 and with five chromosomal fragments identified among the excision plasmids. The data strongly suggest that the integration of pMC7105 may have occurred at a repetitive sequence present on the chromosome and on the plasmid.
Similar content being viewed by others
References
Alwine JC, Kemp DJ, Parker BA, Reiser J, Renart J, Stark GR, Wahl GM (1979) Detection of specific RNA's or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. In: Wu R (ed) Methods in enzymology, vol 68. Academic Press, New York, pp 220–242
Bevan MW, Chilton MD (1982) T-DNA of the Agrobacterium Ti and Ri plasmids. Ann Rev Genet 16:357–384
Bolivar F, Backman K (1979) Plasmids of Escherichia coli as cloning vectors. In: Wu R (ed) Methods in enzymology, vol 68. Academic Press, New York, pp 245–267
Comai L, Kosuge T (1980) Involvement of plasmid deoxyribonucleic acid in indolaecetic acid synthesis in Pseudomonas savastanoi. J Bacteriol 143:950–957
Curiale MS, Mills D (1977) Detection and characterization of plasmids in Pseudomonas glycinea. J Bacteriol 131:224–228
Curiale MS, Mills D (1982) Integration and partial excision of a cryptic plasmid in Pseudomonas syringae pv. phaseolicola. J Bacteriol 152:797–802
Currier TC, Nester EW (1976) Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem 76:431–441
Davis RW, Botstein D, Roth JR (1980) In: Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Dugaiczyk A, Boyer HW, Goodman HM (1975) Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol 96:171–184
Hadley RG, Deonier RC (1980) Specificity in the formation of Atra F-prime plasmids. J Bacteriol 143:680–692
Haas D, Holloway BW (1978) Chromosome mobilization by the R plasmid R6:45: A tool in Pseudomonas genetics Mol Gen Genet 158:229–237
Holloway BW (1979) Plasmids that mobilize bacterial chromosome. Plasmid 2:1–19
Kahn M, Kolter R, Thomas C, Figurski D, Meyer R, Remaut E, Helinski DR (1979) Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. In: Wu R (ed) Methods in enzymology, vol 68. Academic Press, New York, pp 268–280
Lacy GH, Leary JV (1979) Genetic systems in phytopathogenic bacteria. In: Grogan RG, Zentmyer GA, Cowling EB (eds) Ann Rev Phytopathol 17:181–202
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Morrison DA (1979) Transformation and preservation of competent bacterial cells by freezing. In: Wu R (ed) Methods of enzymology, vol 68. Academic Press, New York, pp 326–331
Ohtsubo E, Zenilman M, Ohtsubo H, McCormick M, Machida C, Machida Y (1981) Mechanism of insertion and cointegration mediated by IS1 and Tn3. Cold Spring Harbor Symp Quant Biol 45:283–296
Quant RL, Mills D (1984) An integrative plasmid and multiplesized plasmids of Pseudomonas syringae pv. phaseolicola have extensive homology. Mol Gen Genet 193:459–466
Szabo LJ, Mills D (1984) Involvement of repetitive sequences in the integration of pMC7105 and formation of excision plasmids in Pseudomonas syringae pv. phaseolicola. J Bacteriol 157:821–827
Szabo LJ, Volpe J, Mills D (1981) Identification of F′-like plasmids of Pseudomonas syringae pv. phaseolicola. In: Lozano JC (ed) Proc 5th Int Conf Plant Path Bact, CIAT, Cali, Colombia, pp 403–411
Wilson GA, Young FE (1980) Purification and properties of the BamHI endonuclease. In: Grossman L, Moldave K (ed) Methods of enzymology, vol 65. Academic Press, New York, pp 147–153
Author information
Authors and Affiliations
Additional information
Communicated by A. Böck
Rights and permissions
About this article
Cite this article
Szabo, L.J., Mills, D. Characterization of eight excision plasmids of Pseudomonas syringae pv. phaseolicola . Mol Gen Genet 195, 90–95 (1984). https://doi.org/10.1007/BF00332729
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00332729