Summary
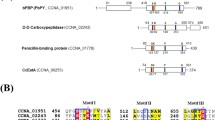
In order to determine the active site of penicillin-binding protein 3 of Escherichia coli (PBP3), the serine residue at position 307 was replaced with alanine, threonine or cysteine by oligonucleotide-directed site-specific mutagenesis. Since a unique BanII site exists at the position corresponding to serine-307, BanII digestion of the plasmid DNA after mutagenesis resulted in significant enrichment of the mutant plasmids. For mutagenesis, the gene coding for PBP3 (ftsI) was inserted into the expression cloning vector pIN-IIB. The hybrid protein produced was able to bind penicillin while mutant PBP3 in which serine-307 was replaced with either alanine or threonine did not lead to any detectable binding. However, contrary to the report of Broome-Smith et al. (1985) thiol-penicillin-binding protein 3, in which serine-307 was replaced with cysteine, was still able to bind penicillin. Replacement of serine-445 with an alanine residue had no effect on penicillin binding to PBP3.
Similar content being viewed by others
References
Broome-Smith JK, Hedge PJ, Spratt BG (1985) Production of thiol-penicillin-binding protein 3 of Escherichia coli using a two primer method of site directed mutagenesis. EMBO J 4: 231–235
Clarke L, Carbon J (1978) Functional expression of cloned yeast DNA in Escherichia coli: specific complementation of argininosuccinate lyase (arg H) mutations. J Mol Biol 120: 517–532
Frère JM, Duez C, Ghuysen JM, Vanderkerckove J (1976) Occurence of a serine residue in the penicillin-binding site of the exocellular DD-carboxy-peptidase-transpeptidase from Streptomyces R61. FEBS Lett 70: 257–260
Glauner B, Keck W, Schwarz U (1984) Analysis of penicillin-binding sites with a micro method — the binding site of PBP 5 from Escherichia coli. FEMS Lett 23: 151–155
Hedge PJ, Spratt BG (1984) A gene fusion that localises the penicillin-binding domain of penicillin-binding protein 3 of Escherichia coli. FEBS Lett 176: 179–184
Inouye S, Inouye M (1985) Oligonucleotide-directed site-specific mutagenesis using double-stranded plasmid DNA. In: Narang S (ed) DNA and RNA synthesis. Academic Press, New York (in press)
Ishino F, Matsuhashi M (1981) Peptidoglycan synthetic enzyme activities of highly purified penicillin-binding protein 3 in Escherichia coli: a septum-forming sequence. Biochem Biophys Res Commun 101: 905–911
Keck W, Glauner B, Schwarz U, Broome-Smith JK, Spratt BG (1985) Sequences of the active-site peptides of three of the high-Mr-penicillin-binding proteins of Escherichia coli K-12. Proc Natl Acad Sci USA 82: 1999–2003
Knott-Hunziker V, Waley SG, Orlek BS, Sammes PG (1979) Penicillinase active sites: labelling of serine-44 in β-lactamase I by 6-bromopenicillanic acid. FEBS Lett 99: 59–61
Knott-Hunziker VS, Petursson GS, Jayatilake GS, Waley SG, Jaurin B, Grundstrom T (1982) Active sites of β-lactamases. Biochem J 201: 621–627
Kraut H, Keck K, Hirota Y (1981) Cloning of ponB and ftsI genes of Escherichia coli and purification of penicillin-binding proteins 1B and 3. Ann Rep Natl Inst Genet 31: 24–29
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Masui Y, Coleman J, Inouye M (1983) Multipurpose expression cloning vehicles in Escherichia coli. In: Inouye M (ed) Experimental manipulation of gene expression. Academic Press, New York, pp 15–32
Matsuhashi M, Nakagawa J, Tomioka S, Ishino F, Tamaki S (1982) Mechanism of peptidoglycan synthesis by penicillin-binding proteins in bacteria and effects of antibiotics. In: Mitsuhashi S (ed) Drug resistance in bacteria — Genetics, biochemistry and molecular biology. Japan Scientific Societies Press, Tokyo, Japan, pp 297–310
Miyoshi K, Arentzen R, Huang T, Itakura K (1980) Solid-phase synthesis of polynucleotides. IV. Usage of polystyrene resins for the synthesis of polydeoxyribonucleotides by the phosphotriester method. Nucleic Acids Res 8: 5507–5517
Morinaga Y, Franceschini T, Inouye S, Inouye M (1984) Improvement of oligonucleotide-directed site-specific mutagenesis using double-stranded plasmid DNA. Bio/Technology 2: 636–639
Nakamura M, Maruyama IN, Soma M, Kato J, Suzuki H, Hirota Y (1983) On the process of cell division in Escherichia coli: Nucleotide sequence of the gene for penicillin-binding protein 3. Mol Gen Genet 191: 1–9
Nicholas RA, Strominger JM, Suzuki H, Hirota Y (1985a) Identification of the active site in penicillin-binding protein 3 of Escherichia coli. J Bacteriol, in press
Nicholas RA, Suzuki H, Hirota Y, Strominger JL (1985b) Purification and sequencing of the active site tryptic peptide from penicillin-binding protein 1b of Escherichia coli. Biochemistry, in press
Nishimura Y, Takeda Y, Nishimura A, Suzuki H, Inouye M, Hirota Y (1977) Synthetic ColE1 plasmids carrying genes for cell division in Escherichia coli. Plasmid 1: 67–77
Rao RN, Rodgers SG (1979) Plasmid pKC7: a vector containing ten restriction endonuclease sites suitable for cloning DNA segments. Gene 7: 79–82
Sancar A, Hack AM, Rupp WD (1979) Simple method for identification of plasmid-coded proteins. J Bacteriol 137: 692–693
Sancar A, Wharton RP, Seltzer S, Kacinski BM, Clarke ND, Rupp WD (1981) Identification of the uvrA gene product. J Mol Biol 148: 45–62
Sigal IS, Harwood BG, Arentzen R (1982) Thiol-β-lactamase: Replacement of the active-site serine of RTEM β-lactamase by a cysteine residue. Proc Natl Acad Sci USA 79: 7157–7160
Sigal IS, De Grado WF, Thomas BJ, Petteway SR Jr (1984) Purification and properties of thiol-β-lactamase, a mutant of pBR322 β-lactamase in which the active site serine has been replaced by cysteine. J Biol Chem 259: 5327–5332
Spratt BG (1977a) Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol 131: 293–305
Spratt BG (1977b) Properties of the penicillin-binding proteins of Escherichia coli K12. Eur J Biochem 72: 341–352
Spratt BG (1983) Penicillin-binding proteins and the future of β-lactam antibiotics. J Gen Microbiol 129: 1247–1260
Spratt BG, Pardee AB (1975) Penicillin-binding proteins and cell shape in E. coli. Nature 254: 516–517
Sugimoto K, Oka K, Sugisaki H, Takanami M, Nishimura A, Yasuda S, Hirota Y (1979) Nucleotide sequence of Escherichia coli K12 replication origin. Proc Natl Acad Sci USA 76: 575–579
Suzuki H, Nishimura Y, Hirota Y (1978) On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci USA 75: 664–668
Waxman D, Strominger JL (1983) Penicillin-binding proteins and the mechanism of action of β-lactam antibiotics. Annu Rev Biochem 52: 825–869
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33: 103–119
Author information
Authors and Affiliations
Additional information
Communicated by M. Takanami
Rights and permissions
About this article
Cite this article
Houba-Hérin, N., Hara, H., Inouye, M. et al. Binding of penicillin to thiol-penicillin-binding protein 3 of Escherichia coli: identification of its active site. Molec Gen Genet 201, 499–504 (1985). https://doi.org/10.1007/BF00331346
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00331346