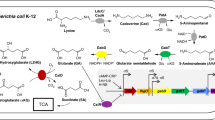
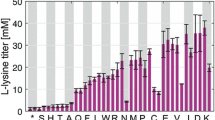
Summary
Stadtman, Holzer and their colleagues (reviewed in Stadtman and Ginsburg 1974) demonstrated that the enzyme glutamine synthetase (GS) [L-glutamate: ammonia ligase (ADP-forming), EC 6.3.1.2] is covalently modified by adenylylation in a variety of bacterial genera and that the modification is reversible. These studies further indicated that adenylylated GS is the less active form in vitro. To assess the physiological significance of adenylylation of GS we have determined the growth defects of mutant strains (glnE) of S. typhimurium that are unable to modify GS and we have determined the basis for these growth defects. The glnE strains, which lack GS adenylyl transferase activity (ATP: [L-glutamate: ammonia ligase (ADP-forming)] adenylyltransferase, EC 2.7.7.42), show a large growth defect specifically upon shift from a nitrogen-limited growth medium to medium containing excess ammonium (NH4 +). The growth defect appears to be due to very high catalytic activity of GS after shift, which lowers the intracellular glutamate pool to ∼10% that under preshift conditions. Consistent with this view, recovery of a rapid growth rate on NH4 + is accompanied by an increase in the glutamate pool. The glnE strains have normal ATP pools after shift. They synthesize very large amounts of glutamine and excrete glutamine into the medium, but excess glutamine does not seem to inhibit growth. We hypothesize that a major function for adenylylation of bacterial GS is to protect the cellular glutamate pool upon shift to NH4 +-excess conditions and thereby to allow rapid growth.
Similar content being viewed by others
References
Alvarez ME, McCarthy CM (1984) Glutamine synthetase from Mycobacterium avium. Can J Microbiol 30:353–359
Bancroft S, Rhee SG, Neumann C, Kustu S (1978) Mutations that alter the covalent modification of glutamine synthetase in Salmonella typhimurium. J Bacteriol 134:1046–1055
Davies W, Ormerod JG (1982) Glutamine synthetase in Chlorobium limicola and Rhodospirillum rubrum. FEMS Microbiol Lett 13:75–78
Dawes I, Mandelstam J (1970) Sporulation of Bacillus subtilis in continuous culture. J Bacteriol 103:529–535
Deuel TF, Stadtman ER (1970) Some kinetic properties of Bacillus subtilis glutamine synthetase. J Biol Chem 245:5206–5213
Donohue TJ, Bernlohr RW (1981) Properties of the Bacillus licheniformis A5 glutamine synthetase purified from cells grown in the presence of ammonia or nitrate. J Bacteriol 147:589–601
Ely B, Amarashinghe AB, Bender RA (1978) Ammonia assimilation and glutamate formation in Caulobacter crescentus. J Bacteriol 133:225–230
Fisher R, Tuli R, Haselkorn R (1981) A cloned cyanobacterial gene for glutamine synthetase functions in Escherichia coli, but the enzyme is not adenylylated. Proc Natl Acad Sci USA 78:3393–3397
Garcia E, Rhee SG (1983) Cascade control of Escherichia coli glutamine synthetase: purification and properties of PII uridylyl-transferase and uridylyl-removing enzyme. J Biol Chem 258:2246–2253
Ginsburg A, Stadtman ER (1973) Regulation of glutamine synthetase in Escherichia coli. In: Prusiner S, Stadtman ER (eds) The enzymes of glutamine metabolism. Academic Press, New York, pp 9–43
Johansson BC, Gest H (1976) Inorganic nitrogen assimilation by the photosynthetic bacterium Rhodopseudomonas capsulata. J Bacteriol 128:683–688
Johansson BC, Gest H (1977) Adenylylation/deadenylylation control of the glutamine synthetase of Rhodopseudomonas capsulata. Eur J Biochem 81:365–371
Kleiner D (1975) Ammonium uptake by nitrogen fixing bacteria. I. Azotobacter vinelandii. Arch Microbiol 104:163–169
Kleiner D, Kleinschmidt JA (1976) Selective inactivation of nitrogenase in Azotobacter vinelandii batch cultures. J Bacteriol 128:117–122
Kleinschmidt JA, Kleiner D (1978) The glutamine synthetase from azotobacter vinelandii: purification, characterization, regulation, and localization. Eur J Biochem 89:51–60
Kustu S, Hirschman J, Meeks JC (1984) Adenylylation of bacterial glutamine synthetase: physiological significance. In: Chock PB, Rhee SG (eds) Molecular basis of cellular regulation. Academic Press, New York, in press
Kustu S, Burton D, Garcia E, McCarter L, McFarland N (1979) Nitrogen control in Salmonella: Regulation by the glnR and glnF gene products. Proc Natl Acad Sci USA 76:4576–4580
Lindroth P, Mopper K (1979) High performance liquid chromatographic determination of subpicomole amounts of amino acids by precolumn fluorescence derivatization with o-phthaldialdehyde. Anal Chem 51:1667–1674
McCarter L, Krajewska-Grynkiewicz K, Trinh D, Wei G, Kustu S (1984) Characterization of mutations that lie in the promoter-regulatory region for glnA, the structural gene encoding glutamine synthetase. Mol Gen Genet, in press
Miflin BJ, Lea PJ, Wallsgrove RM (1980) The role of glutamine in ammonia assimilation and reassimilation in plants. In: Mora J, Palacios R (eds) Glutamine: metabolism, enzymology, and regulation. Academic Press, New York, pp 213–234
Mura U, Chock PB, Stadtman ER (1981) Allosteric regulation of the state of adenylation of glutamine synthetase in permeabilized cell preparations of Escherichia coli. J Biol Chem 256:13022–13029
Orr J, Haselkorn R (1981) Kinetic and inhibition studies of glutamine synthetase from the cyanobacterium Anabaena 7120. J Biol Chem 256:13099–13104
Orr J, Haselkorn R (1982) Regulation of glutamine synthetase activity and synthesis in free-living and symbiotic Anabaena spp. J Bacteriol 152:626–635
Rhee SG, Park R, Chock PB, Stadtman ER (1978) Allosteric regulation of monocyclic interconvertible enzyme cascade systems: Use of Escherichia coli glutamine synthetase as an experimental model. Proc Natl Acad Sci USA 75:3138–3142
Rowell P, Enticott S, Stewart WDP (1977) Glutamine synthetase and nitrogenase activity in the blue-green alga Anbaena cylindrica. New Phytol 79:41–54
Schaeffer P, Millet J, Aubert JP (1965) Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA 54:704–711
Schutt H, Holzer H (1972) Biological function of the ammonia-induced inactivation of glutamine synthetase in Escherichia coli. Eur J Biochem 26:68–72
Senior PJ (1975) Regulation of nitrogen metabolism in Escherichia coli and Klebsiella aerogenes: Studies with the continuous-culture technique. J Bacteriol 123:407–418
Siedel J, Shelton E (1979) Purification and properties of Azotobacter vinelandii glutamine synthetase. Arch Biochem Biophys 192:214–224
Smith CJ, Hespell RB, Bryant MP (1980) Ammonia assimilation and glutamate formation in the anaerobe Selenomonas ruminantium. J Bacteriol 141:593–602
Stacey G, van Baalen C, Tabita FR (1979) Nitrogen and ammonia assimilation in the cyanobacteria: regulation of glutamine synthetase. Arch Biochem Biophys 194:457–467
Stackebrandt E, Woese CR (1981) The evolution of prokaryotes. In: Carlile MJ, Collins JF, Moseley BEB (eds) Molecular and cellular aspects of microbial evolution. Cambridge University Press, pp 1–31
Stadtman ER, Ginsburg A (1974) The glutamine synthetase of Escherichia coli: structure and control. In: Boyer PD (eds) The enzymes, vol 10. Academic Press, New York, pp 755–807
Stadtman ER, Ginsburg A, Ciardi JE, Yeh J, Hennig SB, Shapiro BM (1970) Multiple molecular forms of glutamine synthetase produced by enzyme catalyzed adenylylation and deadenylylation reactions. Adv Enzyme Reg 8:99–118
Stadtman ER, Chock PB (1978) Interconvertible enzyme cascades in metabolic regulation. In: Horecker BL, Stadtman ER (eds) Current topics in cellular regulation, vol 13. Academic Press, New York, pp 53–95
Stadtman ER, Chock PB, Rhee SG (1979) Metabolite control of the glutamine synthetase cascade. In: Atkinson DE, Fox CF (eds) Modulation of protein function. Academic Press, New York, pp 203–217
Stadtman ER, Mura U, Chock PB, Rhee SG (1980) The interconvertible enzyme cascade that regulates glutamine synthetase activity. In: Mora J, Palacios R (eds) Glutamine: metabolism, enzymology, and regulation. Academic Press New York, pp 41–59
Streicher SL, Tyler B (1981) Regulation of glutamine synthetase activity by adenylylation in the Gram-positive bacterium Streptomyces cattleya. Proc Natl Acad Sci USA 78:229–233
Tempest D, Neijssel OM (1979) Eco-physiological aspects of microbial growth in aerobic nutrient-limited environments. In: Alexander M (eds) Advances in microbial ecology, vol 2. Plenum Press, New York, pp 105–153
Tronick SR, Ciardi JE, Stadtman ER (1973) Comparative biochemical and immunological studies of bacterial glutamine synthetases. J Bacteriol 115:858–868
Tumer NE, Robinson SJ, Haselkorn R (1983) Different promoters for the Anabaena glutamine synthetase gene during growth using molecular or fixed nitrogen. Nature 306:337–342
Wedler FC, Hoffman FM (1974) Glutamine synthetase of Bacillus stearothermophilus. I. Purification and basic properties. Biochemistry 13:3207–3214
Wolheuter RM, Schutt H, Holzer H (1973) Regulation of glutamine synthesis in vivo in E. coli. In: Prusiner S, Stadtman ER (eds) The enzymes of glutamine metabolism. Academic Press, New York, pp 45–64
Author information
Authors and Affiliations
Additional information
Communicated by M.M. Green
Rights and permissions
About this article
Cite this article
Kustu, S., Hirschman, J., Burton, D. et al. Covalent modification of bacterial glutamine synthetase: physiological significance. Mol Gen Genet 197, 309–317 (1984). https://doi.org/10.1007/BF00330979
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00330979