Abstract
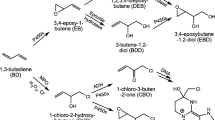
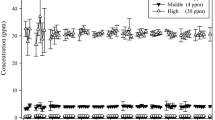
Comparative investigations of inhalation pharmacokinetics of 1,2-epoxybutene-3 (vinyl oxirane, the primary reactive intermediate of butadiene) revealed major differences in metabolism of this compound between rats and mice. Whereas in rats no indication of saturation kinetics of epoxybutene metabolism could be observed up to exposure concentrations of 5000 ppm, in mice saturation of epoxybutene metabolism becomes apparent at atmospheric concentrations of about 500 ppm. The estimated maximal metabolic rate (Vmax) in mice for epoxybutene was only 350 μmol×h−1×kg−1 (rats: >2600 μmol× h−1×kg−1). In the lower concentration range where first order metabolism applies (up to about 500 ppm) epoxybutene is metabolized by mice at higher rates compared to rats (metabolic clearance per kg body weight, mice: 24900 ml×h−1, rats: 13400 ml×h−1). Under these conditions the steady state concentration of epoxybutene in the mouse is about 10 times that in the rat. When mice are exposed to high concentrations of butadiene (>2000 ppm; conditions of saturation of butadiene metabolism; closed exposure system) epoxybutene is exhaled by the animals, and its concentration in the gas phase increases with exposure time. At about 10 ppm epoxybutene signs of acute toxicity are observed. When rats are exposed to butadiene under similar conditions, the epoxybutene concentration reaches a plateau at about 4 ppm. Under these conditions hepatic non-protein sulfhydryl compounds are virtually depleted in mice but not in rats. We conclude that in addition to the higher rate of metabolism of butadiene in mice, limited detoxification and consequently accumulation of its primary reactive intermediate epoxybutene may be a major determinant for the higher susceptibility of mice to butadiene-induced carcinogenesis.
Similar content being viewed by others
References
Bolt HM, Schmiedel G, Filser JG, Rolzhäuser HP, Lieser K, Wistuba D, Schurig V (1983) Biological activation of 1,3-butadiene to vinyl oxirane by rat liver microsomes and expiration of the reactive metabolite by exposed rats. J Cancer Res Clin Oncol 106: 112–116
Bond JA, Dahl AR, Henderson RF, Dutcher JS, Mauderly JL, Birnbaum LS (1986) Species differences in the disposition of inhaled butadiene. Toxicol Appl Pharmacol 84: 617–627
Citti L, Gervasi PG, Turchi G, Belluci G, Bianchini R (1984) The reaction of 3,4-epoxy-1-butene with deoxyguanosine and DNA in vitro: synthesis and characterization of the main adducts. Carcinogenesis 5: 47–52
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82: 70–77
Filser JG, Bolt HM (1979) Pharmacokinetics of halogenated ethylenes in rats. Arch Toxicol 42: 123–136
Filser JG, Bolt HM (1981) Inhalation pharmacokinetics based on gas uptake studies I. Improvement of kinetic models. Arch Toxicol 47: 279–292
Filser JG, Bolt HM (1983) Inhalation pharmacokinetics based on gas uptake studies IV. The endogenous production of volatile compounds. Arch Toxicol 52: 123–133
Filser JG, Bolt HM (1984) Inhalation pharmacokinetics based on gas uptake studies VI. Comparative evaluation of ethylene oxide and butadiene monoxide as exhaled reactive metabolites of ethylene and 1,3-butadiene in rats. Arch Toxicol 55: 219–223
Gervasi PG, Citti L, del Monte M, Longo V, Benetti D (1985) Mutagenicity and chemical reactivity of epoxide intermediates of the isoprene metabolism and other structurally related compounds. Mutat Res 156: 77–82
Hazleton Laboratories Europe (1981) 1,3-butadiene. Inhalation teratogenicity study in the rat. Final report and addendum No. 2788-522/3, Hazleton Labs., Harrowgate HG3 1 PY, England
Huff JE, Melnick RL, Solleveld HA, Hasemann JK, Powers M, Miller RA (1985) Multiple organ carcinogenicity of 1,3-butadiene in B6C3F1 mice after 60 weeks of inhalation exposure. Science 227: 548–549
Kreiling R, Laib RJ, Filser JG, Bolt HM (1986a) Species differences in butadiene metabolism between mice and rats evaluated by inhalation pharmacokinetics. Arch Toxicol 58: 235–238
Kreiling R, Laib RJ, Bolt HM (1986b) Alkylation of nuclear proteins and DNA after exposure of rats and mice to (1,4−14C)1,3-butadiene. Toxicol Lett 30: 131–136
Lorenz J, Glatt HR, Fleischmann R, Ferlinz R, Oesch F (1984) Drug metabolism and its relationship to that in three rodent species: monooxygenase, epoxide hydrolase and glutathione-S-transferase activities in subcellular fractions of lung and liver. Biochem Med 32: 43–56
Schmidt U, Loeser E (1985) Species differences in the formation of butadiene monoxide from 1,3-butadiene. Arch Toxicol 57: 222–225
Van Duuren BL, Langseth L, Orris L, Teebor G, Nelson N, Kuschner M (1966) Carcinogenicity of epoxides, lactones and peroxy compounds IV. Tumor response in epithelial and connective tissue in mice and rats. J Natl Cancer Inst 37: 825–835
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kreiling, R., Laib, R.J., Filser, J.G. et al. Inhalation pharmacokinetics of 1,2-epoxybutene-3 reveal species differences between rats and mice sensitive to butadiene-induced carcinogenesis. Arch Toxicol 61, 7–11 (1987). https://doi.org/10.1007/BF00324541
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00324541