Abstract
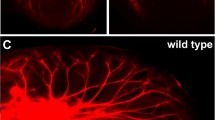
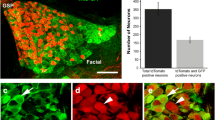
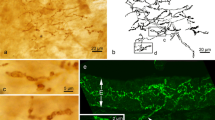
The role of nerve growth factor (NGF) in neurotrophic support for the extrinsic innervation of the nasal and oral mucosae was investigated in keratin 14 (K14)-NGF transgenic mice in which NGF was over-expressed in K14-synthesizing cells. K14 immunoreactivity was localized in the epithelial basal cells of the whisker pad skin, the hard palate, the floor of the ventral meatus, and the anterior tongue that are stratified squamous epithelia, and also in basal cells of the vomeronasal, olfactory, and respiratory epithelia that are non-stratified epithelia. In transgenic mice, NGF expression was identified and confined primarily to the basal cells of stratified epithelia. The nasal mucosae including the vomeronasal, olfactory, and respiratory mucosae, and the glands associated with the vomeronasal organ received a greater innervation of protein gene product 9.5-immunoreactive extrinsic fibers in transgenic animals than nontransgenic controls. An increased density of calcitonin gene-related peptide-immunoreactive extrinsic fibers was observed in the nonsensory epithelia of the vomeronasal organ, the olfactory sensory and respiratory epithelia in transgenic animals. Our results indicated that the hyperinnervation of the nasal and oral mucosae by extrinsic neurons is due at least partially to target-derived NGF synthesis and release by K14-expressing basal cells.
Similar content being viewed by others
References
Albers KM, Wright DE, Davis BM (1994) Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci 14:1422–1432
Arvidsson J, Fundin BT, Pfaller K (1995) Innervation of the hard palate in the rat studied by anterograde transport of horseradish peroxidase conjugates. J Comp Neurol 351:489–498
Barber PC, Raisman G (1978) Cell division in the vomeronasal organ of the adult mouse. Brain Res 141:57–66
Campenot RB (1987) Local control of neurite sprouting in cultured sympathetic neurons by nerve growth factor. Dev Brain Res 37:293–301
Campenot RB (1994) NGF and the local control of nerve terminal growth. J Neurobiol 25:599–611
Conner JM, Muir D, Varon S, Hagg T, Manthorpe M (1992) The localization of nerve growth factor-like immunoreactivity in the adult rat basal forebrain and hippocampal formation. J Comp Neurol 319:454–462
Davies AM, Lumsden AGS (1984) Relation of target encounter and neuronal death to nerve growth factor responsiveness in the developing mouse trigeminal ganglion. J Comp Neurol 253:13–24
Davies AM, Bandtlow C, Heumann R, Korsching S, Rohrer H, Thoenen H (1987) Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature 326:353–358
Davis BR, Albers KM, Seroogy KB, Katz DM (1994) Overex-pression of nerve growth factor in transgenic mice induces novel sympathetic projections to primary sensory neurons. J Comp Neurol 349:464–474
DeLong RE, Getchell TV (1987) Nasal respiratory function-vasomotor and secretory regulation. Chem Senses 12:3–36
Finger TE, Böttger B (1993) Peripheral peptidergic fibers of the trigeminal nerve in the olfactory bulb of the rat. J Comp Neurol 334:117–124
Finger TE, Getchell ML, Getchell TV, Kinnamon JC (1990) Affector and effector functions of peptidergic innervation of the nasal cavity. In: Green BG, Mason JR, Kare MR (eds) Chemical Senses, vol. 2, Irritation. Marcel Denkker, New York, pp 1–20
Galli SJ (1990) Biology of disease. New insights into “the riddle of the mast cells”:Microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab Invest 62:5–33
Getchell ML, Getchell TV (1992) Fine structural aspects of secretion and extrinsic innervation in the olfactory mucosa. Microscopy Res Tech 23:111–127
Getchell ML, Zielinski B, Getchell TV (1988) Odorant and autonomic regulation of secretion in the olfactory mucosa. In: Margolis FL, Getchell TV (eds) Molecular Neurobiology of the Olfactory System. Plenum Press, New York, pp 71–98
Getchell ML, Kulkarmi-Narla A, Takami S, Albers KM, Getchell TV (1995) Age-dependent phenotypic switching of mast cells in NGF-transgenic mice. NeuroReport 6:1261–1266
Graziadei PPC, Monti Graziadei GA (1979) Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol 8:1–18
Horigome K, Pryor JC, Bullock ED, Johnson EM Jr (1993) Mediator release from mast cells by nerve growth factor. Neurotrophin specificity and receptor mediation. J Biol Chem 268:14881–14887
Karanth SS, Springall DR, Kuhn DM, Levene MM, Polak JM (1991) An immunocytochemical study of cutaneous innervation and the distribution of neuropeptides and protein gene product 9.5 in man and commonly employed laboratory animals. Am J Anat 191:369–383
Kinnamon JC, Henzier DM, Royer SM (1993) HVEM ultrastructural analysis of mouse fungiform taste buds, cell types, and associated synapses. Microscopy Res Tech 26:142–156
Korsching S (1993) The neurotrophic factor concept: a reexamination. J Neurosci 13:2739–2748
Levi-Montalcini R (1987) The nerve growth factor 35 years later. Science 237:1154–1162
Moll R, Frank WW, Schiller DL (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11–24
Richardson PM, Riopelle RJ (1984) Uptake of nerve growth factor along peripheral and spinal axons of primary sensory neurons. J Neurosci 4:1683–1689
Sawaf MH, Ouhayoun JP, Shabana AHM, Forest N (1990) Cytokeratin expression in human tongue epithelium. Am J Anat 189:155–166
Schwartz Levey M, Chikaraishi DM, Kauer JS (1991) Characterization of potential precursor populations in the mouse olfactory epithelium using immunocytochemistry and autoradiography. J Neurosci 11:3556–3564
Silver WL (1987) The common chemical sense.In Finger TE, Silver WL (eds) Neurobiology of Taste and Smell. Wiley, New York, pp 65–88
Silverman JD, Kruger L (1989) Calcitonin-gene-related-peptide-immunoreactive innervation of the rat head with emphasis on specialized sensory structures. J Comp Neurol 280:303–330
Stoler A, Kopan R, Duvic M, Fuchs E (1988) Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol 107:427–446
Suzuki Y, Takeda M (1991a) Basal cells in the mouse olfactory epithelium after axotomy: immunohistochemical and electron-microscopic studies. Cell Tissue Res 266:239–245
Suzuki Y, Takeda M (1991b) Keratins in the developing olfactory epithelia. Dev Brain Res 59:171–178
Takami S, Getchell ML, Chen Y, Monti-Bloch L, Berliner DL, Stansaas LJ, Getchell TV (1993) Vomeronasal epithelial cells of the adult human express neuron-specific molecules. Neuro-Report 4:375–378
Takami S, Getchell ML, Getchell TV (1994) Lectin histochemical localization of galactose, N-acetylgalactosamine, and N-acetylglucosamine in glycoconjugates of the rat vomeronasal organ, with comparison to the olfactory and septal mucosae. Cell Tissue Res 277:211–230
Thoenen H (1991) The changing scene of neurotrophic factors. Trends Neurosci 14:165–170
Thompson RJ, Doran JF, Jackson P, Dhillon AP, Rode J (1983) PGP 9.5 — a new marker for vertebrate neurons and neuroendocrine cells. Brain Res 278:224–228
Vaccarezza OL, Sepich LN, Tramezzani JH (1981) The vomeronasal organ of the rat. J Anat 132:167–185
Yamagishi M, Hasegawa S, Takahashi S, Nakano Y, Iwanaga T (1989) Immunohistochemical analysis of the olfactory mucosa by use of antibodies to brain proteins and cytokeratin. Ann Otol Rhinol Laryngol 98:384–388
Yamagishi M, Takami S, Getchell TV (1995) Innervation in human taste buds and its decrease in Alzheimer's disease patients. Acta Otolaryngol (Stockh) (in press)
Young JT (1986) Light microscopic examination of the rat nasal passages: preparation and morphologic features. In: Barrow CS (ed) Toxicology of the Nasal Passages. Hemisphere, Washington, pp 27–36
Author information
Authors and Affiliations
Additional information
This work was supported by NIH grants NIDCD-00159 (T.V.G.), NIDCO-01715 (M.L.G.), and NINDS-31826 (K.M.A.).
Rights and permissions
About this article
Cite this article
Takami, S., Getchell, M.L., Yamagishi, M. et al. Enhanced extrinsic innervation of nasal and oral chemosensory mucosae in keratin 14-NGF transgenic mice. Cell Tissue Res 282, 481–491 (1995). https://doi.org/10.1007/BF00318880
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318880