Summary
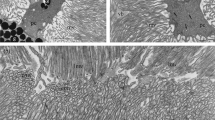
Isolated goldfish retinae were incubated in NaHCO3-reduced solutions, a treatment known to lower intracellular pH and to decrease gap-junction-mediated coupling between cells. The morphology of the gap junctions of horizontal cells examined by means of freezefracture replicas and ultrathin sections displays alterations after such treatment. The gap-junctional particles aggregate into dense clusters or crystalline arrays, whereas controls (pH 7.5) display a loose arrangement of particles. Incubation in NaHCO3-reduced solution leads to the appearance, in ultrathin sections, of prominent, electron-dense material beneath the gap-junctional membranes. Both effects, the increasing density of particles and the appearance of electron-dense material, are reversible. The application of dopamine, which uncouples horizontal cells, and its antagonist haloperidol produce less clear-cut effects on particle density in vitro.
Similar content being viewed by others
References
Aertgeerts PAPM, Scheuermann DW, De Mazière A (1987) Influence of combined hypoxia, hyperkalaemia and substrate depletion on myocardial gap junction structure. Acta Anat 130:3
Baldridge WH, Ball AK, Miller RG (1987) Dopaminergic regulation of horizontal cell gap junction particle density in goldfish retina. J Comp Neurol 265:428–436
Baldridge WH, Ball AK, Miller RG (1989) Gap junction particle density of horizontal cells in goldfish retinas lesioned with 6-OHDA. J Comp Neurol 287:238–246
Bennett MVL, Verselis V, White RL, Spray DC (1988) Gap junctional conductance: gating. In: Hertzberg EL, Johnson R (eds) Gap junctions. Liss, New York, pp 287–304
Boron WF (1983) Transport of H+ and of ionic weak acids and bases. J Membr Biol 72:1–16
Chalcroft JP, Bullivant S (1970) An interpretation of liver cell membrane and junction structure based on observation of freeze-fracture replicas of both sides of the fracture. J Cell Biol 47:49–60
DeVries SH, Schwartz EA (1989) Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol (Lond) 414:351–375
Green CR, Severs NJ (1984) Gap junction connexon configuration in rapidly frozen myocardium and isolated intercalated disks. J Cell Biol 99:453–463
Hanna RB, Spray DC, Model AL, Harris AL, Bennett MVL (1978) Ultrastructure and physiology of gap junctions of an amphibian embryo, effects of CO2. Biol Bull 155:442
Hanna RB, Ornberg RL, Reese TS (1985) Structural details of rapidly frozen gap junctions. In: Bennett MVL, Spray DC (eds) Gap junctions. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 23–32
Hedden WL, Dowling JE (1978) The interplexiform cell system. II. Effects of dopamine on goldfish retinal neurones. Proc R Soc Lond [Biol] 201:27–55
Hidaka S, Shingai R, Dowling JE, Naka K (1989) Junctions form between catfish horizontal cells in culture. Brain Res 498:53–63
Kettenmann H, Schlue W-R (1988) Intracellular pH regulation in cultured mouse oligodendrocytes. J Physiol (Lond) 406:147–162
Kettenmann H, Ransom BR, Schlue W-R (1989) Intracellular pH shifts capable of uncoupling cultured oligodendrocytes are seen only in low HCO -3 solution. Glia 3:110–117
Kirsch M, Wagner H-J (1989) Release pattern of endogenous dopamine in teleost retinae during light adaptation and pharmacological stimulation. Vision Res 29:147–154
Kurz-Isler G, Wolburg H (1986) Gap junctions between horizontal cells in the cyprinid fish alter rapidly their structure during light and dark adaptation. Neurosci Lett 67:7–12
Kurz-Isler G, Wolburg H (1988) Light-dependent dynamics of gap junctions between horizontal cells in the retina of the crucian carp. Cell Tissue Res 251:641–649
Lasater EM (1987) Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cyclic AMP-dependent protein kinase. Proc Natl Acad Sci USA 84:7319–7323
Lasater EM, Dowling JE (1985) Electrical coupling between pairs of isolated fish horizontal cells is modulated by dopamine and cAMP. In: Bennett MVL, Spray DC (eds) Gap junctions. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 393–405
Laufer M, Salas R, Medina R, Drujan B (1989) Cyclic adenosine monophosphate as a second messenger in horizontal cell uncoupling in the teleost retina. J Neurosci Res 24:299–310
Lee WM, Cran DG, Lane NJ (1982) Carbon dioxide induced disassembly of gap junctional plaques. J Cell Sci 57:215–228
Linser P, Moscona AA (1982) Variable CA II compartmentalization in vertebrate retina. Ann NY Acad Sci 429:430–446
Maksimova YM (1970) Effects of intracellular polarization of horizontal cells on the activity of the ganglion cell of the retina of fish. Biofizika 14:570–577
Mangel SC, Dowling JE (1987) The interplexiform-horizontal cell system of the fish retina: effects of dopamine, light stimulation and time in the dark. Proc R Soc Lond [Biol] 231:91–121
Marshak DW, Dowling JE (1987) Synapses of cone horizontal cell axons in goldfish retina. J Comp Neurol 256:430–443
McMahon DG, Knapp AG, Dowling JE (1989) Horizontal cell gap junctions: single-channel conductance and modulation by dopamine. Proc Natl Acad Sci USA 86:7639–7643
Miller TM, Goodenough DA (1985) Gap junction structure after experimental alteration of junctional channel conductance. J Cell Biol 101:1741–1748
Naka K-I (1972) The horizontal cell. Vision Res 12:573–588
Negishi K, Teranishi T, Kato S (1985) Opposite effects of ammonia and carbon dioxide on dye coupling between horizontal cells in the carp retina. Brain Res 342:330–339
Nuccitelli R, Deamer DW (eds) (1982) Intracellular pH: its measurement, regulation and utilization in cellular functions. Liss, New York
Oakley II B, Wen R (1989) Extracellular pH in the isolated retina of the toad in darkness and during illumination. J Physiol (Lond) 419:353–378
Page E, Upshaw-Earley J (1980) Order and dispersal of rat heart gap junctional arrays. J Cell Biol 87:192a
Peracchia C (1980) Structural correlates of gap junction permeation. Int Rev Cytol 66:81–146
Peracchia C, Bernardini G (1984) Gap junction structure and cell-to-cell coupling regulation: is there a calmodulin involvement? Fed Proc 43:2681–2691
Peracchia C, Dulhunty AF (1974) Gap junctions: structural changes assocíated with changes in permeability. J Cell Biol 63:263a
Peracchia C, Peracchia LL (1980) Gap junction dynamics: reversible effects of hydrogen ions. J Cell Biol 87:719–727
Poser K, Kurz-Isler G, Wolburg H, Weiler R (1989) Receptive field properties and intramembrane particle densities of gap junctions between horizontal cells of fish retina. In: Elsner N, Singer W (eds) Dynamics and plasticity in neuronal systems. Thieme, Stuttgart New York, p 324
Ramón y Cajal S (1892) La rétine des vertébrés. La Céllule 9:119–257
Raviola E, Goodenough DA, Raviola G (1980) Structure of rapidly frozen gap junctions. J Cell Biol 87:273–279
Roos A, Boron WF (1981) Intracellular pH. Physiol Rev 61:296–434
Sachs L (1974) Statistische Auswertungsmethoden. Springer, Berlin Heidelberg New York
Seeman P (1987) Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1:133–152
Spray DC, Harris AL, Bennett MVL (1981) Gap junctional conductance is a simple and sensitive function of intracellular pH. Science 211:712–715
Spray DC, Harris AL, Bennett MVL (1982) Comparison of pH and calcium dependence of gap junctional conductance. In: Nuccitelli R, Deamer DW (eds) Intracellular pH: its measurement, regulation and utilization in cellular functions. Liss, New York, pp 445–461
Stell WK (1975) Horizontal cell axons and axon terminals of the goldfish retina. J Comp Neurol 158:503–520
Stell WK, Lightfoot DO (1975) Color-specific interconnections of cones and horizontal cells in the retina of the goldfish. J Comp Neurol 159:473–502
Teranishi T, Negishi K, Kato S (1984) Regulatory effect of dopamine on spatial properties of horizontal cells in carp retina. J Neurosci 4:1271–1280
Turin L, Warner AE (1980) Intracellular pH in early Xenopus embryos: its effect on current flow between blastomeres. J Physiol (Lond) 300:489–504
Webster H deF, Ames III A (1965) Reversible and irreversible changes in the fine structure of nervous tissue during oxygen and glucose deprivation. J Cell Biol 26:885–909
Weiler R (1978) Horizontal cells of the carp retina: Golgi impregnation and procion-yellow injection. Cell Tissue Res 195:515–526
Weiler R, Kohler K, Kolbinger W, Wolburg H, Kurz-Isler G, Wagner-H-J (1988) Dopaminergic neuromodulation in the retinas of lower vertebrates. Neurosci Res [Suppl] 8:183–196
Weiler R, Kolbinger W, Kohler K (1989) Reduced light responsiveness of the cone pathway during prolonged darkness does not result from an increase of dopaminergic activity in the fish retina. Neurosci Lett 99:214–218
Yang X-L, Tornquist K, Dowling JE (1988) Modulation of cone horizontal cell activity in the teleost fish retina. I. Effects of prolonged darkness and background illumination on light responsiveness. J Neurosci 8:2259–2268
Yazulla S (1985) Evoked efflux of [3H] GABA from goldfish retina in the dark. Brain Res 325:171–180
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmitz, Y., Wolburg, H. Gap junction morphology of retinal horizontal cells is sensitive to pH alterations in vitro. Cell Tissue Res 263, 303–310 (1991). https://doi.org/10.1007/BF00318772
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318772