Abstract
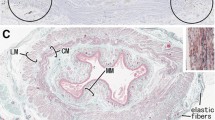
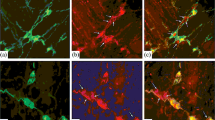
The objective of this study was to examine the effects of two different denervation procedures on the distribution of nerve fibers and neurotransmitter levels in the rat jejunum. Extrinsic nerves were eliminated by crushing the mesenteric pedicle to a segment of jejunum. The myenteric plexus and extrinsic nerves were eliminated by serosal application of the cationic surfactant benzyldimethyltetradecylammonium chloride (BAC). The effects of these two denervation procedures were evaluated at 15 and 45 days. The level of norepinephrine in whole segments of jejunum was initially reduced by more than 76% after both denervation procedures, but by 45 days the level of norepinephrine was the same as in control tissue. Tyrosine hydroxylase (nor-adrenergic nerve marker) immunostaining was absent at 15 days, but returned by 45 days. However, the pattern of noradrenergic innervating axons was altered in the segment deprived of myenteric neurons. Immunohistochemical studies showed protein gene product 9.5 (PGP 9.5)-immunoreactive fibers in whole-mount preparations of the circular smooth muscle in the absence of the myenteric plexus and extrinsic nerves. At 45 days, the number of nerve fibers in the circular smooth muscle increased. Vasoactive intestinal polypeptide (VIP)-immunoreactive fibers, a subset of the PGP 9.5 nerve fibers, were present in the circular smooth muscle at both time points examined. Choline acetyltransferase (CAT) activity and VIP and leucine enkephalin levels were measured in separated smooth muscle and submucosa-musosal layers of the denervated jejunum. VIP and leucine-enkephalin levels were no different from control in tissue that was extrinsically denervated alone. However, the levels of these peptides were elevated two-fold in the smooth muscle 15 and 45 days after myenteric and extrinsic denervation. In the submucosa-mucosa, VIP and leucine enkephalin levels also were elevated two-fold at 15 days, but comparable to control at 45 days. CAT activity was equal to control in the smooth muscle but elevated two-fold in the submucosa-mucosa at both times. These results provide evidence for innervation of the circular smooth muscle by the submucosal plexus. Moreover, these nerve fibers originating from the submucosal plexus proliferate in the absence of the myenteric plexus. Furthermore, the myenteric neurons appear to be essential for normal innervation of the smooth muscle by the sympathetic nerve fibers. It is speculated that the sprouting of the submucosal plexus induced by myenteric plexus ablation is mediated by increased production of trophic factors in the hyperplastic smooth muscle.
Similar content being viewed by others
References
Berthoud HR, Jedrzejewska A, Powley TL (1990) Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with DiI injected into the dorsal vagal complex in the rat. J Comp Neurol 301:65–79
Burnstock G, Evans B, Gannon BJ, Heath JW, James V (1971) A new method of destroying adrenergic nerves in adult animals using guanethidine. Br J Pharmacol 43:295–301
Connors NA, Sullivan JM, Kubb KS (1983) An autoradiographic study of the distribution of fibers from the dorsal motor nucleus of the vagus to the digestive tube of the rat. Acta Anat 115:266–271
Dahl JL, Bloom DD, Epstein ML, Fox DA, Bass P (1987) Effect of chemical ablation of myenteric neurons in neurotransmitter levels in the rat jejunum. Gastroenterology 92:338–344
Domoto T, Bishop AE, Oki M, Polak JM (1990) An in vitro study of the projections of enteric vasoactive intestinal polypeptide immunoreactive neurons in the human colon. Gastroenterology 98:819–827
Downman CBB (1952) Distribution along the small intestine of afferent, vasoconstrictor and inhibitory fibers in the mesenteric nerve bundles. J Physiol (Lond) 116:228–235
Ehrenpreis T (1971) Hirschsprung's disease. Am J Dig Dis 16:1032–1052
Ekblad E, Winther C, Ekman C, Håkanson R, Sundler F (1987) Projections of peptide-containing neurons in rat small intestine. Neuroscience 20:169–188
Epstein ML, Poulsen KT (1991) Appearance of somatostatin and vasoactive intestinal peptide along the developing chicken gut. J Comp Neurol 311:168–178
Ferreira-Santos R, Carril CF (1964) Acquired megacolon in Chagas' disease. Dis Colon Rectum 7:353–364
Fonnum F (1975) A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem 24:407–409
Fox DA, Epstein ML, Bass P (1983) Surfactants selectively ablate enteric neurons of the rat jejunum. J Pharmacol Exp Ther 227:538–544
Furness JB, Costa M (1978) Distribution of intrinsic nerve cell bodies and axons which take up aromatic amines and their precursors in the small intestine of the guinea pig. Cell Tissue Res 188:527–543
Furness JB, Costa M (1987) The enteric nervous system. Churchill Livingstone, London
Furness JB, Lloyd KCK, Sternini C, Walsh JH (1990) Projections of substance P, vasoactive intestinal peptide and tyrosine hydroxylase immunoreactive nerve fibers in the canine intestine, with special reference to the innervation of the circular muscle. Arch Histol Cytol 53:129–140
Gabella G (1984) Size of neurons and glial cells in the intramural ganglia of the hypertrophic intestine of the guinea pig. J Neurocytol 13:73–84
Gershon MD (1981) The enteric nervous system. Ann Rev Neurosci 4:227–272
Herman JR, Bass P (1989) Enteric neuronal ablation: Structure-activity relationship in a series of alkyldimethylbenzylammonium chlorides. Fund Appl Toxicol 13:576–584
Herman JR, Bass P (1990a) Altered carbachol-induced contractile responses of rat jejunal smooth muscle following local myenteric plexus ablation. Dig Dis Sci 35:1146–1152
Herman JR, Bass P (1990b) Pharmacologic characterization of the changes in cholinergic sensitivity of rat jejunum circular muscle following myenteric plexus ablation. J Pharmacol Exp Ther 252:135–139
Heumann R, Koesching S, Scott J, Thoenen H (1984) Relationship between levels of nerve growth factor and its messenger RNA in sympathetic ganglia and peripheral target tissues. EMBO J 3:3183–3189
Hill CE, Hirst GDS, Ngu MC, Heldon DF van (1985) Sympathetic postganglionic reinnervation of mesenteric arteries and enteric neurons of the ileum of the rat. J Auton Nerv Syst 14:317–334
Jacobowitz D (1965) Histochemical studies of the autonomic innervation of the gut. J Pharmacol Exp Ther 149:358–364
Johnson EM, O'Brien F (1976) Evaluation of the permanent sympathectomy produced by the administration of guanethidine to adult rats. J Pharmacol Exp Ther 196:53–61
Kirchgessner AL, Gershon MD (1989) Identification of vagal efferent fibers and putative target neurons in the enteric nervous system of the rat. J Comp Neurol 285:38–53
Krishnamurthy S, Schuffler MD (1987) Pathology of neuromuscular disorders of the small intestine and colon. Gastroenterology 93:610–639
Lander AD (1987) Molecules that make axons grow. Mol Neurobiol 1:231–245
Lindberg I, Smythe SJ, Dahl JL (1979) Regional distribution of enkephalin in bovine brain. Brain Res 168:200–204
Loullis CC, Felten DL, Shea PA (1979) Determination of biogenic amines in discrete brain areas in food deprived rats. Pharmacol Biochem Behav 11:89–93
Lundberg LM, Alm P, Wharton J, Polak JM (1988) Protein gene product 9.5: A new neuronal marker visualizing the whole uterine innervation and pregnancy-induced and developmental changes in the guinea pig. Histochemistry 90:9–17
Nelson DK, Service JE, Studelska DR, Brimijoin S, Go VLW (1988) Gastrointestinal neuropeptide concentrations following guanethidine sympathectomy. J Auton Nerv Syst 22:203–210
Norberg KA (1964) Adrenergic innervation of the intestinal wall studied by fluorescense microscopy. Int J Neuropharmacol 3:379–382
Purves D (1988) Body and brain: A trophic theory of neural connections. Harvard University Press, Cambridge Mass
Purves D, Lichtman JW (1985) Geometrical differences among homologous neurons in mammals. Science 228:298–302
Purves D, Rubin E, Snider WD, Lichtman J(1986) The relation of animal size to convergence, divergence and neuronal number in peripheral sympathetic pathways. J Neurosci 6:158–163
Danders KM, Smith TK (1986) Motoneurones of the submucous plexus regulate electrical activity of the circular muscle of canine proximal colon. J Physiol (Lond) 380:293–310
Schultzburg M, Hökfelt T, Nilsson G, Terenius L, Rehfeld JF, Brown M, Elde R, Goldstein M, Said S (1980) Distribution of peptide and catecholamine containing neurons in the gastrointestinal tract of rat and guinea pig: Immunohistochemical studies with antisera to substance P, vasoactive intestinal polypeptide, enkephalins, somatostatin, gastrin/cholecystokinin, neurotensin and dopamine β-hydroxylase. Neuroscience 5:689–744
See NA, Epstein ML, Schultz E, Pienkowski TP, Bass P (1988) Hyperplasia of jejunal smooth muscle in the myenterically denervated rat. Cell Tissue Res 253:609–617
See NA, Greenwood B, Bass P (1990a) Submucosal plexus alone integrates motor activity and epithelial transport in rat jejunum. Am J Physiol 259 (Gastrointestinal Liver Physiol 22):G593-G598
See NA, Epstein ML, Dahl JL, Bass P (1990b) The myenteric plexus regulates cell growth in rat jejunum. J Auton Nerv Syst 31:219–230
Snider WD (1988) Nerve growth factor enhances dendritic arborization of sympathetic ganglion cells in developing mammals. J Neurosci 8:2628–2634
Steers WD, Ciambolti J, Erdman S, de Groat WC (1990) Morphological plasticity in efferent pathways to the urinary bladder of the rat following urethral obstruction. Neurosci 10:1943–1951
Tennyson VM, Pham TD, Rothman TP, Gershon MD (1986) Abnormalities of smooth muscle, basal laminae and nerves in the aganglionic segments of the bowel of lethal spotted mutant mice. Anat Rec 215:267–281
Thoenen H, Edgar D (1985) Neurotrophic factors. Science 229:238–242
Voyvodic JT (1989) Peripheral target regulation of dendritic geometry in the rat superior cervical ganglion. J Neurosci 9:1997–2010
Zhang X, Fogel R, Simpson P, Renehan W (1991) The target specificity of the extrinsic innervation of the rat small intestine. J Auton Nerv Syst 32:53–62
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Luck, M.S., Dahl, J.L., Boyeson, M.G. et al. Neuroplasticity in the smooth muscle of the myenterically and extrinsically denervated rat jejunum. Cell Tissue Res 271, 363–374 (1993). https://doi.org/10.1007/BF00318623
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318623