Abstract
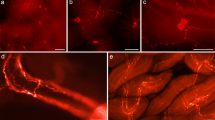
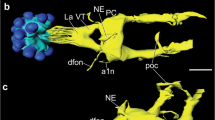
Biogenic peptides and amines associated with the chromaffin tissue in Atlantic cod (Gadus morhua), rainbow trout (Oncorhynchus mykiss), European eel (Anguilla anguilla), spiny dogfish (Squalus acanthias) and Atlantic hagfish (Myxine glutinosa) were identified utilizing immunohistochemical techniques. Within the posterior cardinal vein (PCV) in cod, trout and eel, a subpopulation of chromaffin cells displayed immunoreactivity to tyrosine hydroxylase (TH) and dopamine-β-hydroxylase (DβH) but not to phenylethanolamine-N-methyltransferase (PNMT). TH-like immunorectivity was observed within cells in hagfish hearts. Nerve fibres displaying vasoactive intestinal peptide (VIP) immunoreactivity and pituitary adenylyl cyclase activating peptide (PACAP) immunoreactivity innervated cod, trout and ell chromaffin cells. In eel, neuropeptide Y (NPY)-like and peptide YY (PYY)-like immunoreactivity was located within cells in the PCV, including chromaffin cells. Serotonin-like immunoreactivity was observed within eel and cod chromaffin cells and in hagfish hearts. In the dogfish axillary bodies, nerves displaying TH-like, VIP-like, PACAP-like, substance P-like and galanin-like immunoreactivity were observed. These results are compared with those of other vertebrates, and potential roles for these substances in the control of catecholamine release are suggested.
Similar content being viewed by others
References
Abrahamsson T (1979) Phenylethanolamine-N-methyltransferase (PNMT) activity and catecholamine storage and release from chromaffin tissue of the spiny dogfish, Squalus acanthias. Comp Biochem Physiol [C] 64:169–172
Augustinsson KB, Fänge R, Johnels A, Östlund E (1956) Histological, physiological and biochemical studies on the heart of two cyclostomes, hagfish (Myxine) and lamprey (Lampetra). J Physiol 131:257–276
Bloom G, Östlund E, Euler US von, Lishajko F, Ritzen M, Adams-Ray J (1961) Studies on catecholamine-containing granules of specific cells in cyclostome hearts. Acta Physiol Scand 53 [Suppl 185]:1–34
Delarue C, Leboulenger F, Morra M, Hery F, Verhofstad AJ, Berod A, Denoroy L, Pelletier G, Vaudry H (1988) Immunohistochemical and biochemical evidence for the presence of serotonin in amphibian adrenal chromaffin cells. Brain Res 459:17–26
Delarue C, Becquet D, Idres S, Hery F, Vaudry H (1992) Serotonin synthesis in adrenochromaffin cells. Neuroscience 46:495–500
Euler US von, Fänge R (1961) Catecholamines in nerves and organs of Myxine glutinosa, Squalus acanthias, and Gadus callarias. Gen Comp Endocrinol 1:191–194
Fernandez-Vivero J, Rodriguez-Sanchez F, Verastegui C, Cordoba Moriano F, Romero A, de Castro JM (1993) Immunohistochemical distribution of serotonin and neuropeptide Y (NPY) in mouse adrenal gland. Histol Histopathol 8:509–520
Fried G, Wikstrom LM, Franck J, Rokaeus A (1991) Galanin and neuropeptide Y in chromaffin cells from the guinea-pig. Acta Physiol Scand 142:487–493
Fritsche R, Reid SG, Thomas S, Perry SF (1993) Serotonin-mediated release of catecholamines in the rainbow trout Oncorhynchus mykiss. J Exp Biol 178:191–204
Gallo VP, Civinini A, Mastrolia L, Leitner G, Porta S (1993) Cytological and biochemical studies on chromaffin cells in the head kidney of Gasterosteus aculeatus (Teleostei, Gasteroisteidae). Gen Comp Endocrinol 92:13–142
Grima B, Lamouroux A, Boni C, Julien J-F, Javoy-Agid F, Mallet J (1987) A single human gene encoding multiple tyrosine hydroxylases with different predicted functional characteristics. Nature 326:707–711
Holzwarth MA, Sawetawan C, Brownfield MS (1984) Serotonin-immunoreactivity in the adrenal medulla: distribution and response to pharmacological manipulation. Brain Res Bull 13:299–308
Idres S, Delarue C, Lefebvre H, Vaudry H (1991) Benzamide derivatives provide evidence for the involvement of a 5-HT4 receptor type in the mechanism of action of serotonin in frog adrenocortical cells. Mol Brain Res 10:251–258
Isobe K, Nakai T, Takuwa Y (1993) Ca2+-dependent stimulatory effect of pituitary adenylate cyclase-activating polypeptide on catecholamine secretion from cultured porcine adrenal medullary chromaffin cells. Endocrinology 132:1757–1765
Jönsson AC (1982) Dopamine-β-hydroxylase activity in the axillary bodies, the heart and the splanchnic nerve in two elasmobranchs, Squalus acanthias and Etmopterus spinax. Comp Biochem Physiol [C] 71:191–194
Jönsson AC (1983) Catecholamine formation in vitro in the systemic and portal hearts of the Atlantic hagfish, Myxine glutinosa. Mol Physiol 3:297–304
Johnels AG, Palmgren A (1960) “Chromaffin” cells in the heart of Myxine glutinosa. Acta Zool Bb XLI:314–314
Kuramoto H (1987) An immunohistochemical study of chromaffin cells and nerve fibers in the adrenal gland of the bullfrog Rana catesbeiana. Arch Histol Jpn 50:15–38
Kuramoto H, Kondo H, Fujita T (1985) Substance P-like immunoreactivity in adrenal chromaffin cells and intra-adrenal nerve fibres of rats. Histochemistry 82:507–512
Leboulenger F, Leroux P, Delarue C, Tonon MC, Charnay Y, Dubois PM, Coy DH, Vaudry H (1983a) Co-localization of vasoactive intestinal peptide (VIP) and enkephalins in chromaffin cells of the adrenal gland of Amphibia. Stimulation of corticosteroid production by VIP. Life Sci 32:375–383
Leboulenger F, Leroux P, Tonon MC, Coy DH, Vaudry H, Pelletier G (1983b) Coexistence of vasoactive intestinal peptide and enkephalins in the adrenal chromaffin granules of the frog. Neurosci Lett 37:221–225
Leboulenger F, Cupo A, Castanas E, Benyamina M, Pelletier G, Vaudry H (1986) Immunohistochemical and biochemical evidence for the presence of the pentapeptide met-enkephalin and the heptapeptide met-enkephalin-arg6-phe7 but not the octapeptide met-enkephalin-arg6-gly7-leu8 in amphibian chromaffin cells. Neurochem Int 8:303–309
Lefebvre H, Contesse V, Delarue C, Feuilloley M, Hery F, Grise P, Raynaud G, Verhofstad AAJ, Wolf LM, Vaudry H (1992) Serotonin-induced stimulation of cortisol secretion from human adrenocortical tissue is mediated through activation of a serotonin4 receptor subtype. Neuroscience 47:999–1007
Lundberg JM, Hamberger B, Schultzberg M, Hökfelt T, Granberg PO, Effendic S, Terenius L, Goldstein M, Luft R (1979) Enkephalin- and somatostatin-like immunoreactivities in human adrenal medulla and pheochromocytoma. Proc Natl Acad Sci USA 76:4079–4083
Majane EA, Alho H, Kataoka Y, Lee CH, Yang HYT (1985) Neuropeptide Y in bovine adrenal glands: distribution and characterization. Endocrinology 117:1162–1168
Mastrolia L, Gallo VP, La Marca A (1981) Adrenal homologue in Scardinius erythrophthalmus (Teleosti, Cyprinidae): light and electron microscopic observations. Bool Zool 48:127–138
Mastrolia L, Gallo VP, La Marca A (1984) The adrenal chromaffin cells of Salmo gairdneri Richardson (Teleostei, Salmonidae). J Anat 138:503–511
Masuo Y, Matsumoto Y, Tokito F, Tsuda M, Fujino M (1993) Effects of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) on the spontaneous release of acetylcholine from the rat hippocampus by brain microdialysis. Brain Res 611:207–215
Nandi J (1961) New arrangement of interrenal and chromaffin tissues of teleost fish. Science 134:389–390
Nilsson S (1983) Autonomic nerve function in the vertebrates. In: Farner DS (ed) Zoophysiology, vol 13. Springer, Berlin Heidelberg New York, pp 100–110
Östlund E, Bloom G, Adams-Ray J, Ritzen M, Siegman M, Nordenstam H, Lishajko F, Euler US von (1960) Storage and release of catecholamines, and the occurrence of a specific submicroscopic granulation in hearts of cyclostomes. Nature 188:324–325
Perry SF, Fritsche R, Thomas S (1993) Storage and release of catecholamines from the heart of the Atlantic hagfish, Myxine glutinosa. J Exp Biol 183:165–184
Reinecke M, Heym C, Forssmann WG (1992) Distribution patterns and coexistence of neurohormonal peptides (ANP, BNP, NPY, SP, CGRP, enkephalins) in chromaffin cells and nerve fibres of the anuran adrenal organ. Cell Tissue Res 268:247–256
Steiner HJ, Schmid KW, Fisher-Colbrie R, Sperk G, Winkler H (1989) Co-localization of chromogranin A and B, secretogranin II and neuropeptide Y in chromaffin granules of rat adrenal medulla studied by electron microscopic immunohistochemistry. Histochemistry 91:473–477
Viveros OH, Wilson SP (1983) The adrenal chromaffin cell as a model to study the co-secretion of enkephalins and catecholamines. J Auton Nerv Syst 7:41–58
Viveros OH, Diliberto EJ, Hazum E, Chang KJ (1980) Enkephalins as possible adrenomedullary hormones: storage, secretion and regulation of synthesis. In: Costa E, Trabucchi M (eds) Neural peptides and neural communications. Raven Press, New York, pp 191–204
Wakade TD, Blank MA, Malhorta RK, Pourcho R, Wakade AR (1991) The peptide VIP is a neurotransmitter in rat adrenal medulla: physiological role in controlling catecholamine secretion. J Physiol 444:349–362
Watanabe T, Masuo Y, Matsumoto H, Suzuki N, Ohtaki T, Masuda Y, Kitada C, Tsuda M, Fujino M (1992) Pituitary adenylate cyclase activating polypeptide provokes cultured rat chromaffin cells to secrete adrenaline. Biochem Biophys Res Comm 182:403–411
Yamaguchi N (1993) In vivo evidence for adrenal catecholamine release mediated by nonnicotinic mechanism: local medullary effect of VIP. Am J Physiol 265:R766-R771
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reid, S.G., Fritsche, R. & Jönsson, AC. Immunohistochemical localization of bioactive peptides and amines associated with the chromaffin tissue of five species of fish. Cell Tissue Res 280, 499–512 (1995). https://doi.org/10.1007/BF00318354
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318354