Summary
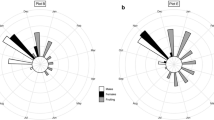
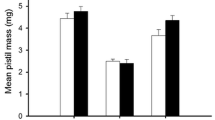
Baccharis halimifolia (Compositae) is a dioecious shrub which grows on the upland fringe of tidal marshes along the Atlantic and Gulf Coasts of North America. We examined the responses of the two sexes to variation in nutrient and moisture availability plant density, and defoliation. By growing plants from seedlings to flowering adults under various combinations of soil type, fertilization rate and plant density, we were able to establish different rates of plant growth and mortality. Plants grown at high density and low nutrient and water supply grew the least, incurrent the most mortality and showed a male-biased sex ratio (73% male). At low density with abundant nutrients and water, plants grew more, survived well, flowered frequently, and were female-biased (75% female). Changes in sex ratio were probably the result of sex-related mortality rather than sexual lability of the seedlings. While changes in sex ratio occurred under experimental conditions in the green-house, no evidence for differences in habitat utilization between the sexes were found in the field and the sex ratio (59% female) did not vary across habitats. In the marsh habitats we sampled where water and nutrients were apparently available, there was no evidence for differential mortality between the sexes. When defoliated (75% of leaf tissue), both sexes showed similar reductions in reproductive effort (number of flower heads/shoot). Our results indicate that differences between the sexes of Baccharis in their response to environmental growing conditions is an important ecological factor associated with the separation of male and female function into separate individuals.
Similar content being viewed by others
References
Baker HG (1959) Reproductive methods as factors in specialization in flowering plants. Cold Spring Harbor Symp Quant Biol 24:177–191
Bawa KS (1980) Evolution of dioecy in flowering plants. Ann Rev Ecol Syst 11:15–39
Bierzychudek P (1981) The demography of jack-in-the-pulpit, a forest perennial that changes sex. Ph. D. Dissertation, Cornell University, Ithaca, New York
Bierzychudek P, Eckhart V (1988) Spatial segregation of the sexes of dioecious plants. Am Nat 132:34–43
Boldt PE (1989) Biology and host specificity of Trihabda bacaridis (Coleoptera: Chrysomelidae) on Baccharis (Asteraceae: Asterae). Environ Entomol 18:76–84
Bullock S, Bawa KS (1891) Sexual dimorphism and annual flowering pattern in Jacaratia dolichaula (D. Smith) Woodson (Caricaceae) in a Costa Rican rainforest. Ecology 64:851–861
Charnov EL (1982) The theory of sex allocation. Monograph in population biology, Vol. 18. Princeton University Press, Princeton, New Jersey
Cronquist A (1980) Vascular flora of the Southeastern United States. University of North Carolina Press, Chapel Hill, North Carolina
Dawson TE, Bliss LC (1989) Patterns of water use and the tissue water relations in the dioecious shrub, Salix arctica: the physiological basis for habitat partitioning between the sexes. Oecologia 79:332–343
Freeman DC, Klickoff LG, Harper KT (1976) Differential resource utilization by the sexes of dioecious plants. Science 193:597–599
Freeman DC, Harper KT, Charnov EL (1980) Sex change in plants: Old and new observations and new hypotheses. Oecologia 47:222–232
Freeman DC, McArthur ED (1982) A ocmparison of water stress between males and females of six species of desert shrubs. For Sci 28:304–308
Krischik VA (1984) The role of temporal and spatial variability in leaf nitrogen, water, toughness, and resin content in the interaction between Trirhabda bacaridis (Weber) (Coleoptera: Chrysomelidae) and its host Baccharis halimifolia. PH.D. Dissertation, University of Maryland, College, Park, Maryland
Krischik VA, Denno RF (1983) Individual, population, and geographic patterns in plant defense. In: Denno RF, McClure MS (eds) Academic Variable Plants and Herbivores in Natural and Managed Systems. Academic Press, New York, pp 463–512
Lloyd DG, Bawa KS (1984) Modification of the gender of seed plants in varying conditions. In: Hecht MK, Wallace B, Prance GT (eds) Evolutionary Biology vol 17, Plenum Press, New York, pp 255–335
Lloyd DG, Webb CJ (1977) Secondary sex characteristics in seed plants. Bot Rev 43:177–216
Meagher TR (1980) The population biology of Chamaelirium luteum, a dioecious lily I. Spatial distribution of males and females. Evolution 34:1127–1137
Meagher TR (1981) Population of Chamaelirium luteum, a dioecious lily II. Mechanism governing sex ratios. Evolution 35:557–567
Meagher TR (1984) Sexual dimorphism and ecological differentiation of male and female plants. Ann Misso Bot Gard 71:254–264
Meagher TR, Antonovics J (1982a) Life history variation in dioecious plant populations: a case study of Chamaelirium luteum. In: Dingle H, Hegmann JP (eds) Evolution and Genetics of Life Histories, Springer-Verlag, New York, pp 139–154
Meagher TR, Antonovics J (1982b) The population biology of Chamaelirium luteum, a dioecious member of the lily family III. Life history studies. Ecology 63:1690–1700
Onyekweln SS Harper JL (1979) Sex ratio and niche differentation in spinach (Spinacia oleracea L.). Nature 282:609–611
Policansky D (1981) Sex choice and the size advantage model in jack-in-the-pulpit (Arisaema triphyllum) Proc Natl Acad Sci USA 78:1306–1308
Policansky D (1982) Sex change in plants and animals. Ann Rev Ecol Syst 13:471–495
SAS Institute Inc. SAS Users Guide (1985) Cary, North Carolina: SAS Institute Inc.
Thompson JD, Barrett SCH (1981) Selection for outcrossing, sexual selection and the evolution of dioecy in plants. Am Nat 118:443–449
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aber Krischik, V., Denno, R.F. Differences in environmental response between the sexes of the dioecious shrub Baccharis halimifolia (Compositae). Oecologia 83, 176–181 (1990). https://doi.org/10.1007/BF00317749
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317749