Summary
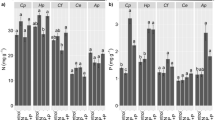
We studied nitrate reductase (NR) activity in six species of the genus Piper (Piperaceae) growing under a broad range of light availabilities. Field measurements were made on plants growing naturally in rainforest at the Los Tuxtlas Tropical Biological Preserve, Veracruz, Mexico at high- and lowlight extremes for each species. Foliar nitrogen on an area basis was positively related to the average daily photosynthetically active photon flux density (PFD) received by the leaf (r=0.76, p<0.01). In vivo NR activity was highly correlated with PFD (r=0.95, p<0.001) and less so with total leaf nitrogen (r=0.68, p<0.05). In vivo NR activity was always higher in high-light plants than in low-light plants within a species. Similarly, gap species such as P. auritum had much higher in vivo NR activities than shade species such as P. aequale. Soil NO −3 and NH +4 pools and nitrogen-mineralization rates at Los Tuxtlas were similar between high- and low-light sites, indicating that the elevated NR activities in high-light plants were not the result of higher NO −3 availabilities in high-light microsites. We performed additional experiments at Stanford, California, USA on Piper plants grown at high- and low-light. Foliar NR was highly inducible by nitrate in the gap species (auritum) but not in the generalist (hispidum) or shade (aequale) species. Root NR activities were, in general, an order of magnitude lower than foliar activities. In total, these studies suggest that Piper gap species are inherently more competent to assimilate NO −3 and are better able to respond to sudden increases in NO −3 availability than are shade species.
Similar content being viewed by others
References
Andrews M (1986) The partitioning of nitrate assimilation between root and shoot of higher plants. Plant Cell Environ 9:511–519
Bazzaz FA, Carlson RW (1982) Photosynthetic acclimation to variablility in the light environment of early and late successional plants. Oecologia 53:313–316
Beevers L, Hageman RH (1983) Uptake and reduction of nitrate: Bacteria and higher plants. In: Lauchli A, Bieleski RL (eds), Encyclopedia of plant physiology, NS, vol 15A, Springer, Berlin Heidelberg New York, pp 351–357
Binkley D, Vitousek PM (1989) Soil nutrient availability. In Pearcy RW, Ehleringer JR, Mooney HA, Rundel PW (eds), Plant physiological ecology: Field methods and instrumentation, Chapman Hall, London New York, pp 75–96
Björkman O (1972) Photosynthetic adaptation to contrasting light climates. Carneg Instit Wash Yearb 71:82–135
Björkman O (1981) Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds), Encyclopedia of plant physiology, NS, vol 12A, Springer, Berlin Heidelberg New York, pp 57–107
Campbell WH (1988) Nitrate reductase and its role in nitrate assimilation in plants. Phys Plant 74:214–219
Chapin FS III, Bloom AJ, Field CB, Waring RH (1987) Interactions of environmental factors in controlling plant growth. Bio-Science 37:49–57
Chazdon RL, Fetcher N (1984) Photosynthetic light environments in a lowland tropical rain forest in Costa Rica. J Ecol 72:553–564
Chazdon RL, Field CB (1987a) Determinants of photosynthetic capacity in six rainforest Piper species. Oecologia 73:222–230
Chazdon RL, Field CB (1987b) Photographic estimation of photosynthetically active radiation: evaluation of a computerized technique. Oecologia 73:525–532
Estrada A, Coates-Estrada R, Martínez-Ramos M (1985) La Estación de Biologia Tropical Los Tuxtlas: un recurso para el estudio y conservación de la selvas del trópico húmedo. In: Gómez-Pompa A, del Amo S (eds) Investigaciones sobre la Regeneración de Selvas en Veracruz, Mexico, vol II, INIREB, Alhambra, Mexico, pp 379–393
Gebauer G, Rehder H, Wollenweber B (1988) Nitrate, nitrate reduction and organic nitrogen in plants from different ecological and taxonomic groups of Central Europe. Oecologia 75:371–385
Gómez-Pompa, A (1971) Posible papel de la vegetación secundaria en la evolución de la flora tropical. Biotropica 3:125–135
Gutschick VP 1981 Evolved strategies in nitrogen acquisition by plants. Amer Nat 118:607–637
Harper JE, Hageman RH (1972) Canopy and seasonal profiles of nitrate reductase in soybeans (Glycine max [L.] Merr.). Plant Physiol 49:146–154
Isaac RA, Johnson WC (1976) Determination of total nitrogen in plant tissue using a block digestor. J Assoc Anal Chem 59:98–100
Lee JA, Stewart GR (1978) Ecological aspects of nitrogen assimilation. Adv Bot Res 6:1–43
Leegood RC, Walker DA, Foyer CH (1985) Regulation of the Benson-Calvin cycle. In: Barber J, Baker NR (eds), Photosynthetic mechanisms and the environment, Elsevier Science Publishers, Cambridge, pp 191–258
Lillo C, Henriksen A (1984) Comperative studies of diurnal variations of nitrate reductase activity in wheat, oat, and barley. Physiol Plant 62:89–94
Martínez-Ramos M, Alvarez-Buylla E, Sarukhán J, Piñero D (1988) Treefall age determination and gap dynamics in a tropical forest. J Ecol 76:700–716
Matson PA, Vitousek PM, Ewel JJ, Mazzarino MJ (1987) Nitrogen transformations following tropical forest felling and burning on a volcanic soil. Ecol 68(3):491–502
Nicholas JC, Harper JE, Hageman RH (1976) Nitrate reductase activity in soybeans (Glycine max [L.] Merr.). II. Energy limitations. Plant Physiol 58: 736–739
Pate JS (1980) Transport and partitioning of nitrogenous solutes. Ann Rev Plant Physiol 31:313–340
Pate JS (1986) Economy of symbiotic nitrogen fixation. In: Givnish TJ (ed), On the economy of plant form and function, Cambridge Univ Press, Cambridge, pp 299–325
Peet MM, Raper DC, Tolley LC, Robarge WP (1985) Tomato responses to ammonium and nitrate nutrition under controlled root-zone pH. J Plant Nutr 8:787–798
Robertson CP (1984) Nitrification and nitrogen mineralization in a lowland rainforest in Costa Rica, Central America. Oecologia 61:91–104
Rzedowski J (1978) Vegetacion de Mexico, Editorial Limusa, Mexico
Scholl RL, Harper JE, Hageman RH (1974) Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol 53: 825–828
Smirnoff N, Stewart GR (1985) Nitrate assimilation and translocation by higher plants: Comparative physiology and ecological consequences. Physiol Plant 64:133–140
Smirnoff N, Todd P, Stewart GR (1984) The occurrence of nitrate reduction in the leaves of woody plants. Ann Bot 54:363–374
Stewart GR, Popp M, Holzapfel I, Stewart JA, Dickie-Eskew A (1986) Localization of nitrate reduction in ferns and its relationship to environment and physiological characteristics. New Phytol 104:373–384
Stewart G, Hegarty EE, Specht RL (1988) Inorganic nitrogen assimilation in plants of Australian rainforest communities. Phys Plant 74:26–33
Vazquez-Yanes C (1976) Estudios sobre ecophysiologia de la germación en una zona calido-húmeda de Mexico. In: Gomez-Pompa A, Vazquez-Yanes C, Del Amo S, Butanda A (eds), Regeneración de selvas, Editorial Continental, Mexico, pp 279–381
Vitousek PM, Denslow JS (1986) Nitrogen and phosphorus avialability in treefall gaps of a lowland tropical rainforest. J Ecol 74:1167–1178
Vitousek PM, Sanford RL Jr (1986) Nutrient cycling in moist tropical forests. Ann Rev Ecol Syst 17:137–167
Walters MB, Field CB (1987) Photosynthetic acclimation in two rainforest Piper species with different ecological amplitudes. Oecologia 72:449–456
Author information
Authors and Affiliations
Additional information
CJWDPB publication # 1097
Rights and permissions
About this article
Cite this article
Fredeen, A.L., Griffin, K. & Field, C.B. Effects of light quantity and quality and soil nitrogen status on nitrate reductase activity in rainforest species of the genus Piper . Oecologia 86, 441–446 (1991). https://doi.org/10.1007/BF00317614
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317614