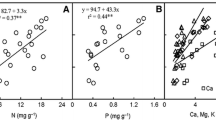
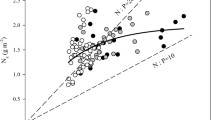
Abstract
Partitioning of nitrogen among species was determined in a stand of a tall herbaceous community. Total amount of nitrogen in the aboveground biomass was 261 mmol N m−2, of which 92% was in three dominant species (Phragmites, Calamagrostis and Carex) and the rest was in the other eight subordinate species. Higher nitrogen concentrations per unit leaf area (n L) with increasing photosynthetically active photon flux density (PPFD) were observed in all species except for three short species. The changes in n L within species were mainly explained by the different nitrogen concentrations per unit leaf mass, while the differences in n L between species were explained by the different SLM (leaf mass per unit leaf area). Photon absorption per unit leaf nitrogen (Φ N ) was determined for each species. If photosynthetic activity was proportional to photon absorption, Φ N should indicate in situ PNUE (photosynthetic nitrogen use efficiency). High Φ N of Calamagrostis (dominant) resulted from high photon absorption per unit leaf area (Φ area ), whereas high Φ N of Scutellaria (subordinate) resulted from low n L although its Φ area was low. Species with cylinder-like “leaves” (Juncus and Equisetum) had low Φ N , which resulted from their high n L. Light-saturated CO2 exchange rates per unit leaf area (CER) and per unit leaf nitrogen (potential PNUE) were determined in seven species. Species with high CER and high n L (Phragmites, Carex and Juncus) had low potential PNUE, while species with low CER and low n L showed high potential PNUE. NUE (ratio of dry mass production to nitrogen uptake) was approximated as a reciprocal of plant nitrogen concentration. In most species, three measures of nitrogen use efficiency (NUE, Φ N and potential PNUE) showed strong conformity. Nitrogen use efficiency was high in Calamagrostis and Scutellaria, intermediate in Phragmites and relatively low in Carex. Nitrogen use efficiency of subordinate species was as high as or even higher than that of dominant species, which suggests that growth is co-limited by light and nitrogen in the subordinate species.
Similar content being viewed by others
References
Ackerly DD (1992) Light, leaf age, and leaf nitrogen concentration in a tropical vine. Oecologia 89:596–600
Acock B, Charles-Edwards DA, Fitter DJ, Hand DW, Ludwig LJ, Warren Wilson J, Withers AC (1978) The contribution of leaves from different levels within a tomato crop to canopy net photosynthesis: an experimental examination of two canopy models. J Exp Bot. 29:815–827
Aerts R (1994) Effects of nitrogen supply on canopy structure and leaf nitrogen distribution in Carex species. Ecology 75: 1482–1490
Berendse F, Aerts R (1987) Nitrogen-use-efficiency: a biologically meaningful definition? Funct Ecol 1: 293–296
Björkman O (1982) Responses to different quantum flux densities. In: Lange OL Nobel PS, Osmond CB, Ziegler H (ed) Physiological plant ecology I (Encyclopedia of plant physiology Vol 12A). Springer, Berlin Heidelberg New York pp 57–107
Bloom AJ, Chapin III FS, Mooney HA (1985) Resource limitation in plants — an economic analogy. Annu Rev Ecol Syst 16: 363–392
Chapin FS III (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11: 233–260
DeJong TM, Doyle JF (1985) Seasonal relationships between leaf nitrogen content of leaves from different horizons within a canopy. Plant Cell Environ 8: 701–706
De Wit CT, Dijkshoorn W, Noggle JC (1963) Ionic balance and the growth of plants. Verslagen Landbouwkundig Onderzoek 69, Wageningen
Ellsworth DS, Reich PB (1993) Canopy structure and vertical patterns of photosynthesis and related leaf traits in a deciduous forest. Oecologia 96: 169–178
Evans J (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19
Field CB (1983) Allocating leaf nitrogen for the maximization of carbon gain: leaf age as a control on the allocation program. Oecologia 56: 348–355
Field CB, Mooney HZ (1986) The photosynthesis-nitrogen relationship in wild plants. In: Givnish TJ (ed) On the economy of plant form and function. Cambridge University Press, Cambridge, pp 25–55
Gulmon SL, Chu CC (1981) The effects of light and nitrogen on photosynthesis, leaf characteristics, and dry matter allocation in the chaparral shurb, Diplacus aurantiacus. Oecologia 49: 207–212
Hikosaka K, Terashima I, Katoh S (1994) Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Oecologia 97: 451–457
Hirose T (1975) Relations between turnover rate, resource utility and structure of some plant populations: a study in the matter budgets. J Fac Sci Univ Tokyo III, 11: 355–407.
Hirose T, Werger MJA (1987a) Nitrogen use efficiency in instantaneous and daily photosynthesis of leaves in the canopy of a Solidago altissima stand. Physiol Plant 70: 215–222
Hirose T, Werger MJA (1987b) Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 72: 520–526
Hirose T, Werger MJA (1994) Canopy structure and photon flux partitioning among species in a herbaceous plant community. Ecology (in press)
Hirose T, Werger MJA, Pons TL, Rheenen JWA van (1988) Canopy structure and leaf nitrogen distribution in a stand of Lysimachia vulgaris L. as influenced by stand density. Oecologia 77: 145–150
Hirose T, Werger MJA, Rheenen JWA van (1989) Canopy development and leaf nitrogen distribution in a stand of Carex acutiformis. Ecology 70: 1610–1618
Makino A, Nakano H, Mae T (1994) Responses of ribulose-1,5-bisphosphate carboxylase, cytochrome f, and sucrose synthesis enzymes in rice leaves to leaf nitrogen and their relationships to photosynthesis. Plant Physiol 105: 173–179
Monsi M, Saeki T (1953) Über de Lichtfaktor in den Pflanzenge-sellschaften und seine bedeutung für die Stoffproduktion. Jpn J Bot 14: 22–52
Nevins DJ, Loomis RS (1970) Nitrogen nutrition and photosynthesis in sugar beet (Beta vulgaris L.) Crop Sci 10: 21–25
Pons TL, Schieving F, Hirose T, Werger MJA (1989) Optimization of leaf nitrogen allocation for canopy photosynthesis in Lysimachia vulgaris. In: Lambers H, Cambridge M, Konings H, Pons TL (eds) Causes and consequences of variation in growth rate and productivity of higher plants. SPB Academic, The Hague, pp 175–186
Pons TL, Rijnberk H van, Scheurwater I, Werf A van der (1993) Importance of the gradient in photosynthetically active radiation in a vegetation stand for leaf nitrogen allocation in two monocotyledons. Oecologia 95: 416–424
Schieving F, Pons TL, Werger MJA, Hirose T (1992) The vertical distribution of nitrogen and photosynthetic activity at different plant densities in Carex acutiformis. Plant Soil 142: 9–17
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. Freeman, San Francisco
Van der Meijden R (1990) Heukel's flora van Nederland, 21st edn. Wolters-Noordhoff, Groningen
Verhoeven JTA (ed) (1992) Fens and bogs in the Netherlands: vegetation, history, nutrient dynamics and conservation. Kluwer, Dordrecht
Verhoeven JTA, Beek S van, Dekker M, Storm W (1983) Nutrient dynamics in small mesotrophic fens surrounded by cultivated land. I. Productivity and nutrient uptake by the vegetation in relation to the flow of eutrophicated ground water. Oecologia 60: 25–33
Vitousek P (1982) Nutrient cycling and nutrient use efficiency. Am Nat 119: 553–572
Werger MJA, Hirose T (1988) Effects of light climate and nitrogen partitioning on the canopy structure of stands of a dicotyledonous, herbaceous vegetation. In: Werger MJA, Aart PJM van der, During HJ, Verhoeven JTA (eds) Plant form and vegetation structure. SPB Academic, The Hague, pp 171–181
Werger MJA, Hitose T (1991) Leaf nitrogen distribution and whole canopy photosynthetic carbon gain in herbaceous stands. Vegetatio 97: 11–20
Westhoff V, Den Held AJ (1969) Plantengemeenschappen in Nederland. Thieme, Zutphen
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hirose, T., Werger, M.J.A. Photosynthetic capacity and nitrogen partitioning among species in the canopy of a herbaceous plant community. Oecologia 100, 203–212 (1994). https://doi.org/10.1007/BF00316946
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00316946