Summary
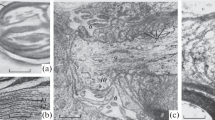
Morphological aspects of myelin breakdown in the posterior funiculus during Wallerian degeneration were studied in kittens subjected to lumbosacral dorsal rhizotomies 6–8 days after birth. The first sign of myelin breakdown was characterized by swollen or shrunken nerve fibers. Shortly thereafter there was an increased occurrence of collapsed myelin sheaths and later of rounded myelin bodies. Myelin was clearly seen in microglial cells. Correlative observations on Marchi-stained material indicated the simultaneous and frequent appearance of Marchi-positive bodies (MPB:s) and myelin bodies. Due to the rapidity of the degeneration process in the kitten, the increase in the occurrence of Marchi-positive granules (MPG:s) seemed to start concomitantly with increased occurrence of MPB:s. However, the frequent occurrence of MPG:s outlasted that for MPB:s. The findings indicate that the MPB:s may be the counterpart to myelin bodies and the MPG:s to lipid droplets. Microglial cells may be responsible for the primary uptake of degenerating myelin and the subsequent transformation of myelin bodies to lipid droplets. The much faster breakdown of myelin and elimination of lipid material in the degenerating posteror funiculus of the kitten, as compared to the adult, seemed to be due not only to the lower myelin content in the kitten, but also to a higher density of microglia and a greater efficiency in the myelin breakdown process in the degenerating posterior funiculus of the kitten.
Similar content being viewed by others
References
Adams CWM (1958) Histochemical mechanisms of the Marchi reaction for degenerating myelin. J Neurochem 2:178–186
Adams CWM (1960) Osmiumtetroxide and the Marchi method: Reactions with polar and non-polar lipids, protein and polysacharide. J Histochem Cytochem 8:262–267
Adams CWM (1965) Neurohistochemistry. Elsevier, New York
Adams CWM, Bayliss OB (1968) Reappraisal of osmium tetroxide and OTAN histochemical reactions. Histochemie 16:162–166
Aldskogius H (1974) Indirect and direct Wallerian degeneration in the intramedullary root fibres of the hypoglossal nerve. An electron microscopical study in the kitten. Advances in Anatomy Embryology and Cell Biology, vol. 50. Springer, Berlin Heidelberg New York
Berthold C-H, Nilsson I (1987) Redistribution of Schwann cells in developing feline L7 ventral spinal roots. J Neurocytol 16:811–828
Cook RD (1974) Observations on glial cells within myelin sheaths in degenerating optic nerves. J Neurocytol 3:737–751
Cook RD, Ghetti B, Wisniewski HM (1974) The pattern of Wallerian degeneration in the optic nerve of newborn kittens: An ultrastructural study. Brain Res 75:261–275
Corneliuson O, Berthold C-H, Fabricius C, Gatzinsky K, Carlstedt T (1988a) Marchi-positive myelinoid bodies at the transition between the central and the peripheral nervous system in some vertebrates. J Anat (in press)
Corneliuson O, Berthold C-H, Persson H, Fredman P (1988b) Aspects on the protein and the lipid composition of myelinoid Marchi-positive bodies from mammalian spinal cord. Neurochem Res (in press)
Daniel PM, Strich SJ (1969) Histological observations on Wallerian degeneration in the spinal cord of the baboon, Papio Papio. Acta Neuropathol (Berl) 12:314–328
Elleder M, Lojda Z (1968a) Remarks on the detection of osmium derivates in tissue sections. Histochemie 13:276–282
Elleder M, Lojda Z (1968b) Remarks on the “OTAN” reaction. Histochemie 14:47–64
Franson P (1985) Quantitative electron microscopic observations on the non-neuronal cells and lipid droplets in the posterior funiculus of the cat after dorsal rhizotomy. J Comp Neurol 231:490–499
Franson P (1988) Quantitative electron microscopic observations on the non-neuronal cells and lipid droplets in the posterior funiculus of the kitten after dorsal rhizotomy. Anat Embryol 178:95–105
Franson P, Ronnevi L-O (1984) Myelin breakdown and elimination in the posterior funiculus of the adult cat after dorsal rhizotomy: A light and electron microscopic qualitative and quantitative study. J Com Neurol 223:138–151
Fulcrand J, Privat A (1977) Neuroglial reactions secondary to Wallerian degeneration in the optic nerve of the postnatal rat: Ultrastructural and quantitative study. J Comp Neurol 176:189–224
Hildebrand C (1971a) Ultrastructural and light-microscopic studies of the developing feline spinal cord white matter. 1. The nodes of Ranvier. Acta Physiol Scand [Suppl] 364:81–108
Hildebrand C (1971b) Ultrastructural and light microscopic studies of the developing feline spinal cord white matter. II. Cell death and myelin sheath disintegration in the early postnatal period. Acta Physiol Scand [Suppl] 364:109–145
Hildebrand C (1977) Presence of Marchi-positive myelinoid bodies in the spinal cord white matter of some vertebrate species. J Morphol 153:1–22
Hildebrand C (1982) Electron microscopic identification of Gomori positive rings in normal spinal cord white matter. Acta Neuropathol 56 (1):29–34
Hildebrand C, Aldskogius H (1976) Electron-microscopic identification of Marchi-positive bodies and argyrophilic granules in the spinal cord white matter of the guinea pig. J Comp Neurol 170:191–203
Mugnaini E, Walberg F (1967) An experimental electron microscopical study on the mode of termination of cerebellar corticovestibular fibres in the cat lateral vestibular nucleus (Deiter's nucleus). Exp Brain Res 4:212–236
Remahl S, Risling M, Hildebrand C (1977) Age-related changes in occurrence of Marchi-positive granules and Marchi-positive myelinoid bodies in postnatally developing feline white matter. J Neurol Sci 34:71–86
Ronnevi L-O (1978) Origin of the glial processes responsible for the spontaneous postnatal phagocytosis of boutons on cat spinal motoneurons. Cell Tissue Res 189:203–217
Scott PP, da Silva AC, Lloyd-Jacob MA (1957) The cat. In: Worden AN, Lane-Petter W (eds) The UFAW handbook on the care and management of laboratory animals. UFAW, London
Spatz von H (1921) Über die Vorgänge nach experimenteller Rückenmarksdurchtrennung mit besonderer Berücksichtigung der Unterschiede der Reaktionsweise des reifen und des unreifen Gewebes-nebst Beziehungen zur menschlichen Pathologie (Porenzephalie und Syringomyelie). Nissl.-Alzheimers histologische und histopathologische Arbeit über der Grosshirnrinde, pp 49–364 (Summary, pp 306–315)
Stoll G, Trapp BD, Griffin JW (1988) Macrophage function during Wallerian degeneration of rat optic nerve: Clearance of degenerating myelin and IA expression. Society for neuroscience, Abstracts, vol 14, part 1. 18th Ann Meet, Toronto-Ontario (November 1988)
Strich S (1968) Notes on the Marchi method for staining degenerating myelin in the peripheral and central nervous system. J Neurol Neurosurg Psychiatr 31:110–114
Swank RL, Davenport H (1935) Chlorate-osmic-formalin method for staining degenerating myelin. Stain Technol 10:87–90
Weibel ER (1973) Stereological techniques for electron microscopic morphometry. In: Hayat MA (ed) Principles and Techniques of Electron Microscopy, Biological Applications, vol 3. Van Nostrand Reinhold Company, Melbourne, pp 237–298
Wolfgram F, Rose AS (1958) Chemical basis of the Marchi method for degenerating myelin. Neurology (Minneap) 8:839–841
Wolfgram F (1956) Study of the early changes occurring in degenerating myelin. Neurology (Minneap.) 6:636–639
Wolman M (1957) Histochemical study of changes occurring during the degeneration of myelin. J Neurochem 1:370–376
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Franson, P., Ronnevi, LO. Myelin breakdown in the posterior funiculus of the kitten after dorsal rhizotomy. Anat Embryol 180, 273–280 (1989). https://doi.org/10.1007/BF00315885
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315885