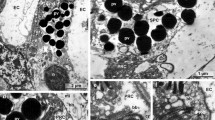
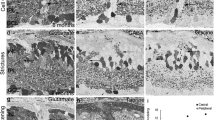
Summary
Retinal differentiation in the pouch young of the wallaby Macropus eugenii was characterized microscopically and morphometrically. Mitosis occurs until early in the second month in the central retina, and until early in the fourth month, peripherally. Separation of the neuroblast layer by the outer plexiform layer did not immediately halt cell division. The retinal surface continued to expand well past the time of cessation of proliferation. Cell death in the ganglion cell layer continued through the fourth month centrally and to nearly five months in the periphery. The major period of cell death was coincident with the segregation of retinal afferents and the refinement of topography in the superior colliculus and dorsal lateral geniculate necleus. Beginning in the third month retinal thickness, measured between the outer limiting membrane and nerve fiber layer declined equally in peripheral and central regions. At all stages the combined thicknesses of the outer and inner nuclear layer in the retinal periphery was greater than that in the center. Together with a late thickening of the inner plexiform layer, the data are consistent with the suggestion that expansion of peripheral non-ganglion cell elements may play a role in development of center to periphery differences in ganglion cell distributions.
Retinal differentiation of the wallaby follows the pattern of most mammals. The onset of development of key milestones for the acquisition of retinal function occurred in the sequence: conventional synapse formation prior to ribbon synapse formation in the inner plexiform layer, and photoreceptor outer segment differentiation prior to terminal triad synapse formation.
Similar content being viewed by others
References
Beazley LD, Dunlop SA (1983) The evolution of an area centralis and visual streak in the marsupial Setonix brachyurus. J Comp Neurol 216:211–231
Beazley LD, Perry VH, Baker B, Darby JE (1987) An investigation into the role of ganglion cells in the regulation of division and death of other retinal cells. Dev Brain Res 33:169–184
Beazley LD, Dunlop SA, Harman AM (1988) Close encounters with developing eyes and brains. Aust Soc Exp Biol Proc 61–64
Blanks JC, Adinolfi AM, Lolley RN (1974) Photoreceptor degeneration and synaptogenesis in retinal-degenerative (rd) mice. J Comp Neurol 156:95–106
Braekevelt CR, Hollenberg MJ (1970) Development of the retina of the albino rat. Am J Anat 127:281–302
Chalupa LM, Williams RW (1984) Prenatal development and reorganization in the visual system of the cat. In: Stone J, Dreher B, Rapaport DH (eds) Development of Visual Pathways in Mammals. Alan Liss, New York, pp 89–102
Cowan WM, Fawcett JW, O'Leary DDM, Stanfield BB (1984) Regressive events in neurogenesis. Science 225:1258–1265
Cragg BG (1975) The development of synapses in the visual system of the cat. J Comp Neurol 160:147–166
Dreher B, Potts RA, Ni SYK, Bennett MR (1984) The development of heterogeneities in distribution and soma sizes of rat retinal ganglion cells. In: Stone J, Dreher B, Rapaport DH (eds) Development of Visual Pathways in Mammals. Alan Liss, New York, pp 39–57
Dunlop SA, Beazley LD (1985) Changing distribution of retinal ganglion cells during area centralis and visual streak formation in the marsupial Setonix brachyurus. Brain Res 355:81–90
Dunlop SA, Beazley LD (1987) Cell death in the developing retinal ganglion cell layer of the wallaby Setonix brachyurus. J Comp Neurol 264:14–23
Dunlop SA, Longley WA, Beazley LD (1987) Development of the area centralis and visual streak in the grey kangaroo Macropus fuliginosus. Vision Res 27:151–164
Fisher LJ (1976) Synaptic arrays of the inner plexiform layer in the developing retina of Xenopus. Dev Biol 50:402–412
Fry KR (1983) Development of the guinea pig retina. Ph D Dissertation. The University of Calgary, Calgary, Alberta
Greiner JV, Weidman TA (1980) Histogenesis of the cat retina. Exp Eye Res 30:439–454
Greiner JV, Weidman TA (1982) Embryogenesis of the rabbit retina. Exp Eye Res 34:749–765
Grun G (1977) The ultrastructural differentiation of synaptic sites in the inner plexiform layer of a teleostan retina. Z Mikrosk Anat Forsch 91:687–703
Guilford JB, Fruchter B (1978) Fundamental statistics in psychology and education, 6th edn. McGraw-Hill, New York
Harman AM, Beazley LD (1987) Patterns of cytogenesis in the developing retina of the wallaby Setonix brachyurus. Anat Embryol 177:123–130
Hendrickson A, Kupfer C (1976) The histogenesis of the fovea in the macaque monkey. Invest Ophthal Vis Sci 15:746–756
Hollenberg MJ, Spira AW (1973) Human retinal development: Ultrastructure of the outer retina. Am J Anat 137:357–387
Hughes WF, LaVelle A (1974) On the synaptogenic sequence in the chick retina. Anat Rec 179:297–301
Jeffrey G (1984) Retinal ganglion cell death and terminal field retraction in the developing rodent visual system. Dev Brain Res 13:81–96
Lia B, Williams RW, Chalupa LM (1987) Formation of retinal ganglion cell topography during prenatal development. Science 236:848–851
Marotte L (1988) Development of topography of retinal connections to the dorsal lateral geniculate and superior colliculus of the wallaby. Neurosci Lett [Suppl] 30:S 96
Marotte LR, Mark RF (1988) Retinal projections to the superior colliculus and dorsal lateral geniculate nucleus in the tammar wallaby (Macropus eugenii): II. Topography after rotation of an eye prior to retinal innervation of the brain. J Comp Neurol 271:274–292
Mastronarde DN, Thibeault MA, Dubin MW (1984) Non-uniform postnatal growth of the cat retina. J Comp Neurol 228:598–608
McArdle CB, Dowling JE, Masland RH (1977) Development of outer segments and synapses in the rabbit retina. J Comp Neurol 175:253–273
Meller K (1964) Elektronenmikroskopische Befunde zur Differenzierung der Rezeptorzellen und Bipolarzellen der Retina und ihrer synaptischen Verbindungen. Z Zellforsch 64:733–750
Meller K, Tetzlaff W (1976) Scanning electron microscopic studies on the development of the chick retina. Cell Tissue Res 170:145–160
Merchant JC (1979) The effect of pregnancy on the interval between one oestrus and the next in the tammar wallaby, Macropus eugenii. J Reprod Fertil 56:459–464
Moyer F (1961) Electron microscopic observations on the origin, development and genetic control of melanin granules in the mouse eye. In: Smelser GK (ed) The Structure of the Eye. Academic Press, New York, pp 469–486
Nelson J (1987) The early development of the eye of the pouch-young of the marsupial Dasyurus hallucatus. Anat Embryol 175:387–398
Nishimura Y, Rakic P (1985) Development of the rhesus monkey retina. I. Emergence of the inner plexiform layer and its synapses. J Comp Neurol 241:420–434
O'Day K (1936) A preliminary note on the presence of double cone and oil droplets in the retina of marsupials. J Anat 70:465–467
O'Leary DDM, Fawcett JW, Cowan WM (1986) Topographic targeting errors in the retinocollicular projection and their elimination by selective ganglion cell death. J Neurosci 6:3692–3705
Olney JW (1968) An electron microscopic study of synapse formation, receptor outer segment development, and other aspects of developing mouse retina. Invest Ophthal Vis Sci 7:250–268
Provis JM (1987) Patterns of cell death in the ganglion cell layer of the human fetal retina. J Comp Neurol 259:237–246
Provis JM, van Driel D, Billson FA, Russell P (1985) Development of the human retina: Patterns of cell distribution and redistribution in the ganglion cell layer. J Comp Neurol 233:429–451
Rapaport DH, Stone J (1982) The site of commencement of maturation in mammalian retina: observations in the cat. Brain Res 5:273–279
Rapaport DH, Stone J (1983) The topography of cytogenesis in the developing retina of the cat. J Neurosci 3:1824–1834
Rapaport DH, Robinson SR, Stone J (1984) Cell movement and birth in the developing cat retina. In: Stone J, Dreher B, Rapaport DH (eds) Development of visual pathways in mammals. Alan R Liss, New York, pp 23–38
Robinson SR (1987) Ontogeny of the area centralis in the cat. J Comp Neurol 255:50–67
Robinson SR (1988) Cell death in the inner and outer nuclear layers of the developing cat retina. J Comp Neurol 267:507–515
Samorajski T, Keffe JR, Ordy JM (1965) Morphogenesis of photoreceptor and retinal ultrastructure in a sub-human primate. Vision Res 5:639–648
Sanyal S, Bal AK (1973) Comparative light and electron microscopic study of retinal histogenesis in normal and rd mutant mice. Z Anat Entwickl Gesch 142:219–238
Sengelaub DR, Finlay BL (1982) Cell death in the mammalian visual system during normal development: I. Retinal ganglion cells. J Comp Neurology 204:311–317
Sengelaub DR, Dolan PR, Finlay BL (1986) Cell generation, death, and retinal growth in the development of the hamster retinal ganglion cell layer. J Comp Neurol 246:527–543
Smelser GK, Ozanics V, Rayborn M, Sagun D (1974) Retinal synaptogenesis in the primate. Invest Ophthal Vis Sci 13:340–362
Spira AW (1975) In utero development and maturation of the retina of a non-primate mammal: a light and electron microscopic study of the guinea pig. Anat Embryol 146:279–300
Spira AW, Hollenberg MJ (1973) Human retinal development: Ultrastructure of the inner retinal layers. Dev Biol 31:1–21
Spira AW, Marotte LR (1987) Development of the retina in the wallaby (Macropus eugenii). Neurosci Lett [Suppl] 27:S 128
Spira AW, Hudy S, Hannah RS (1984) Ectopic photoreceptor cells and cell death in the developing rat retina. Anat Embryol 169:293–301
Stone J, Egan M, Rapaport DH (1985) The site of commencement of retinal maturation in the rabbit. Vision Res 25:309–317
Stone J, Maslim J, Rapaport D (1984) The development of the topographical organisation of the cat's retina. In: Stone J, Dreher B, Rapaport DH (eds) Development of visual pathways in mammals. Alan R Liss, New York, pp 3–21
Stone J, Rapaport DH, Williams RW, Chalupa L (1982) Uniformity of cell distribution in the ganglion cell layer of prenatal cat retina: Implications for mechanisms of retinal development. Dev Brain Res 2:231–242
Strongin AC, Guillery RW (1981) The distribution of melanin in the developing optic cup and stalk and its relation to cellular degeneration. J Neurosci 1:1193–1204
Tancred E (1981) The distribution and sizes of ganglion cells in the retinas of five Australian marsupials. J Comp Neurol 196:585–603
Tokuyasu K, Yamada E (1959) The fine structure of the retina studied with the electron microscope. IV. Morphogenesis of outer segments of retinal rods. J Biophys Biochem Cytol 6:225–230
Vogel M (1978) Postnatal development of the cat's retina. Adv Anat Embryol Cell Biol 54:1–66
Vollmer G, Layer PG (1986) Reaggregation of chick retinal and mixtures of retinal and pigment epithelial cells: the degree of laminar organization is dependent on age. Neurosci Lett 63:91–95
Walls GL (1963) The Vertebrate Eye and Its Adaptive Radiation. Hafner Publ, New York
Weidman TA, Kuwabara T (1968) Postnatal development of the rat retina. An electron microscopic study. Arch Ophthal 79:470–484
Weidman TA, Kuwabara T (1969) Development of the rat retina. Invest Ophthal Vis Sci 8:60–69
Wong RO, Wye-Dvorak J, Henry GH (1986) Morphology and distribution of neurons in the retinal ganglion cell layer of the adult tammar wallaby — Macropus eugenii. J Comp Neurol 253:1–12
Wye-Dvorak J (1984) Postnatal development of primary visual projections in the tammar wallaby (Macropus eugenii). J Comp Neurol 228:491–508
Yamada E, Ishikawa T (1965) Some observations of the submicroscopic morphogenesis of the human retina. In: Rohen J (ed) The structure of the eye. Schattauer, Stuttgart, pp 5–16
Yew DT (1979) Cell kinetic in retinal cell morphogenesis. Neuroscience Lett 13:173–176
Young RW (1984) Cell death during differentiation of the retina in the mouse. J Comp Neurol 229:362–373
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spira, A.W., Marotte, L.R. Histological and electron microscopic milestones in the development of the retina of a marsupial wallaby, Macropus eugenii . Anat Embryol 179, 571–585 (1989). https://doi.org/10.1007/BF00315699
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315699