Summary
In a double-blind, placebo-controlled, crossover experiment in 21 healthy male volunteers, aged 19 to 27 y, the pharmacokinetics and tolerance of the new anxiolytic drug alpidem (SL80.0342) and its three major metabolites were studied after single doses of 25, 50, 100 and 200 mg. Plasma concentrations of alpidem (in 20 subjects) and metabolites (in 6 subjects) were measured by HPLC over a period of 54 h after dosing.
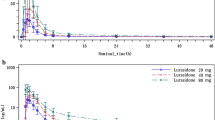
Cmax, tmax and AUC(0–54) and, when possible, t1/2 were determined for alpidem and metabolites and the dose linearity of the parameters was investigated. The time to peak of alpidem was dose independent in most subjects and was short (1–4 h); the mean values at the four dosing levels were 1.9, 1.7, 1.6 and 1.8 h. The peak concentration increased with the dose, the mean values being 17, 34, 88 and 115 ng · ml−1, respectively. In 50% of the subjects cmax tended to stabilize between the 100 and 200 mg dose. Dose linearity was also present for the AUC, which plateaued between the 100 and 200 mg dose in only 3 out of 20 subjects; the mean AUC was 119, 281, 669 and 1117 ng · ml−1 · h, respectively. The apparent half-life of elimination appeared to be dose independent, mean values at the increasing dosing levels being 18.7, 19.9, 18,1 and 17.9 h.
A similar relationship between the kinetics parameters and dose of the alpidem was observed for the metabolites SL83.0912, SL80.0522 and SL83.0725. The formation of metabolites was not saturated as their AUCs relative to corresponding alpidem AUCs were not dose related.
Thus the kinetics of alpidem and its three major metabolites were linear after doses of 25 to 200 mg.
The drug was well tolerated by most of the subjects. Sedation and dizziness occurred mainly after the 100 and 200 mg doses.
Similar content being viewed by others
References
Ascalone V, Catalani P, Dal Bo' L, Deschamps N, Guinebault P (1987) Determination of Alpidem and its metabolites in human plasma by high performance liquid chromatography and fluorimetric detection. J Chromatogr Biomed Appl 414: 101–108
Curran HV, Allen D, Lader M (1987) The effects of single doses of Alpidem and Lorazepam in memory and psychomotor performance in normal humans. J Psychopharmacol 1: 65–73
Dimsdale M, Friedman JC, Morselli PL, Zivkovic B (1988) Alpidem. Drugs Future 13: 106–109
Hindmarch I, Shillingford CA, Shillingford J, Baksi A, Graham D, Guillet P (1988) The pharmacokinetic effects of Alpidem in elderly subjects. J Drug Dev 1: 77–85
Lader M (1988) Clinical Pharmacology of non-benzodiazepine anxiolytics, Pharmacol Biochem Behav 29: 797–798
Langer SF, Feighber JP, Pambokian R, Manowitz NR, Venable PC (1988) Pilot study on alpidem, a novel imidazopyridine compound, in anxiety. Psychopharmacol Bull 24: 161–163
Langer SZ, Arbilla S (1988) Imidazopyridines as a tool for the characterization of benzodiazepine receptors: a prosposal for a pharmacological classification as omega receptors subtypes. Pharmacol Biochem Behav 29: 763–766
Langer SZ, Arbilla S, Tan S, Lloyd KG, George P, Allen J, Wick AE (1990) Selectivity for omega-receptor subtypes as a strategy for the development of anxiolytic drugs. Pharmacopsychiatry 23 (Suppl III): 103–107
Lo MMS, Snijder SH (1983) Two distinct solubilized benzodiazepine receptors: differential modulation by ions. J Neurochem 3: 2270–2279
Musch B, Morselli PL, Priore P (1988) Clinical studies with the new anxiolytic alpidem in anxious patients: an overview of European experiences. Pharmacol Biochem Behav 29: 803–806
Pacifici GM, Bianchetti G, Viani A, Rizzo G, Carrai M, Allen J, Morselli PL (1989) Binding of alpidem, a new anxiolytic agent, to human plasma proteins. Eur J Clin Pharmacol 37: 29–32
Padovani P, Guinebault P, Vajta S, Durand A, Allen J, Thenot JP, Morselli PL (1987) A preliminary metabolic study of alpidem in rat and man. Eur J Drug Metab Pharmacokinet 12: 295–298
Saletu B, Grumberger J, Linzmager L (1986a) Pharmacokinetic and dynamic studies with a new anxiolytic imidazopyridine alpidem utilizing EEG and psychometry. Int Clin Physiopharm 1: 145–164
Saletu B, Schuller M, Grumberger J, Chaudry HR (1986b) Sleep laboratory study of a new anti anxiety drug, alpidem: short term trial. Curr Ther Res 40: 769–779
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jonkman, J.H.G., Bianchetti, G., Grasmeijer, G. et al. Clinical pharmacokinetics and tolerability of alpidem in healthy subjects given increasing single doses. Eur J Clin Pharmacol 41, 369–374 (1991). https://doi.org/10.1007/BF00314970
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00314970