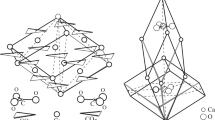
Abstract
Ar, N2 and CO2 were introduced into the structural cavities of channel-evacuated single-crystals of White Well cordierite with the composition:
K0.01Na0.03(Mg1.91Fe0.09Mn0.01)Al3.98Si5.01O18. The gas refilling experiments were carried out in conventional hydrothermal bombs at 6–7 kbar and 600–700°C. The increase in the mean refractive indices for gas-treated crystals, as determined with a spindle-stage equipped microscope, was used along with point-dipole calculations to estimate the percentage of occupied structural cavities. The steep increase of the electronic polarizability parallel to the a-axis, which can be derived from the increase of the refractive index n γ (Z∥a) upon introduction of volatiles, indicates that N2 and CO2 are preferentially aligned parallel to the a-axis of cordierite. Single-crystal structure refinements at room temperature confirm these predictions. Additionally, decreased C–O and N–N bond lengths suggest a librational motion with a mean rotary oscillation angle of 35° (N2) and 25° (CO2) about a, where c is the rotation axis. Mean libration angles of 40° (N2) and 28° (CO2) were estimated from the electronic polarizability tensors of CO2 and N2. Site occupancy refinements of the channel position are in good agreement with the optically derived values for the volatile concentrations, both indicating about 70% and 60% filled cavities for Ar- and N2-cordierite, respectively. Chemical analyses and point-dipole calculations confirm that about 45% of the cavities are occupied in the CO2-treated crystal. The structural framework of cordierite is slightly but specifically altered by the various channel occupants.
Similar content being viewed by others
References
Aines RD, Rossman GR (1984) The high temperature behavior of water and carbon dioxide in cordierite and beryl. Am Mineral 69:319–327
Aranovich LYa, Podlesskii KK, Shchepochkina NI (1981) Experimental determination of the solubility of carbon dioxide in cordierite (in russ.) Doklady Akademii Nauk SSSR Moscva 261:728–730
Armbruster Th, Bloss FD (1980) Channel CO2 in cordierite. Nature 236:140–141
Armbruster Th, Bloss FD (1982) Orientation and effects of channel H2O and CO2 in cordierite. Am Mineral 67:284–291
Armbruster Th, Bürgi HB (1982) Orientation of CO2 in the cavity of cordierite, a single crystal X-ray study at 100, 300, and 500 K. Fortschr Mineral 60 Beih 1:37–39
Armbruster Th, Schreyer W, Hoefs J (1982) Very high CO2 cordierite from Norwegian Lapland: mineralogy, petrology, and carbon isotopes. Contrib Mineral Petrol 81:262–267
Beltrame RJ, Norman DI, Alexander EC, Sawkins FJ (1976) Volatiles released by step-heating a cordierite to 1,200°C. Trans Am Geophys Union 57:352
Bloss FD (1981) The spindle stage, principles and practice. Cambridge University Press, Cambridge and New York
Born M (1972) Optik. Springer-Verlag, Berlin Heidelberg New York
Busing WR, Levy HA (1964) The effect of thermal motion on the estimation of bond lengths from diffraction measurements. Acta Crystallogr 17:142–146
Carson DG, Rossman GR, Vaughan RW (1982) Orientation and motion of water molecules in cordierite: a proton nuclear magnetic resonance study. Phys Chem Miner 8:14–19
Cohen JM, Ross FK, Gibbs GV (1977) An X-ray and neutron diffraction study of hydrous low cordierite. Am Mineral 62:67–78
Cruickshank DWJ (1956) Errors in bond lengths due to rotational oscillation of molecules. Acta Crystallogr 9:757–758
Damon PE, Kulp JL (1958) Excess helium and argon in beryl and other minerals. Am Mineral 43:433–459
Donohue J (1961) Refinement of the positional parameter in α-nitrogen. Acta Crystallogr 14:1000–1001
Dunitz JD (1979) X-ray analysis and the structure of organic molecules. Cornell University Press, Ithaca
Enraf Nonius (1983) Structure determination package (SDP), Enraf Nonius, Delft
Evans RC (1966) An introduction to crystal chemistry, Cambridge University Press, Cambridge
Farmer VC (1974) The infrared spectra of minerals. Miner Soc London
Farrel F, Newnham RE (1967) Electronic and vibrational absorption spectra in cordierite. Am Mineral 52:380–388
Gibbs GV (1966) The polymorphism of cordierite I: the crystal structure of low cordierite. Am Mineral 51:1068–1087
Gies H (1983) Studies on clathrasils III. Crystal structure of melanophlogite, a natural clathrate compound of silica. Z Kristallogr 164:247–257
Goldman DS, Rossman GR, Dollase WA (1977) Channel constituents in cordierite. Am Mineral 62:1144–1157
Hartshorne NH, Stuart A (1960) Crystals and the polarizing microscope, Edward Arnold, London
Henshaw DG (1958) Atomic distribution in liquid and solid neon and solid argon by neutron diffraction. Phys Rev 111:1470–1475
Herzberg G (1955) Spectra of diatomic molecules. London
Hochella MF, Brown EB, Ross FK, Gibbs GV (1979) High-temperature crystal chemistry of hydrous Mg- and Fe-cordierites. Am Mineral 64:337–351
International Tables for X-ray Crystallography (1962) Vol IV, Kynoch Press Birmingham
Johannes W, Schreyer W (1981) Experimental introduction of CO2 and H2O into Mg-cordierite. Am J Sci 281:299–317
Karle IL, Karle J (1949) Internal motion and molecular structure studies by electron diffraction. J Chem Phys 17:1052–1058
Kittel C (1973) Einführung in die Festkörperphysik. R. Oldenbourg München, Wien, John Wiley and Sons Frankfurt
Langer K, Schreyer W (1969) Infrared and powder X-ray diffraction studies on the polymorphism of cordierite. Am Mineral 54:1442–1459
La Placa SJ, Hamilton WC (1972) Refinement of the crystal structure of α-N2. Acta Crystallogr B28:984–985
Lepezin GG, Melenevsky VN (1977) On the problem of water diffusion in the cordierites. Lithos 10:49–57
Medenbach O, Maresch WV, Mirwald PW, Schreyer W (1980) Variation of refractive index of synthetic Mg-cordierite with H2O content. Am Mineral 65:367–373
Mottana A, Fusi A, Potenza BB, Crespi R, Liborio G (1983) Hydrocarbon-bearing cordierite from the Dervio-Colico road tunnel (Como, Italy). N Jahrb Miner Abh 148:181–199
Norman DI, Alexander EC, Sawkins FJ (1976) Helium in cordierites: a possible indicator of low temperature metamorphic events. Trans Am Geophys Union 57:352
Pauling L (1968) Die Natur der chemischen Bindung. Verlag Chemie Weinheim/Bergstr
Pohl D (1978) Electronic polarizabilities of ions in double refracting crystals. Acta Crystallogr A34:574–578
Pryce M (1973) Low iron cordierite in phlogopite schist from White Well, Western Australia. Mineral Mag 39:241–243
Schreyer W, Yoder HS, Aldrich LT (1960) Synthesis of an argoncontaining cordierite. Ann Rep Dir Geophys Lab 59:94–96
Simon A, Peters K (1980) Single-crystal refinement of the structure of carbon dioxide. Acta Crystallogr B36:2750–2751
Smith JV, Schreyer W (1962) Location of argon and water in cordierite. Mineral Mag 33:226–236
Stewart JM, Machin PA, Dickinson CW, Ammon HL, Heck H, Flack H (1976) The X-ray system of crystallographic programs, version 1976. Technic. Rep. Tr. 446, Computer Science Center, Univ. Maryland, College Park, Maryland
Venables JA, English CA (1974) Electron diffraction and the structure of α-N2. Acta Crystallogr B30:929–935
Wallace JH, Wenk HR (1980) Structure variation in low cordierites. Am Mineral 65:96–111
Weast RC (ed) (1974) Handbook of Chemistry and Physics. CRC Press, Cleveland, Ohio
Werding G, Mirwald PW (1982) Infrarotspektren CO2-haltiger Mg-Cordierite. Bunsentagung Ulm 1982 Abstract C25
Wood DL, Nassau K (1967) Infrared spectra of foreign molecules in beryl. J Chem Phys 47:2220–2228
Zimmermann JL (1981) La libération de l' eau, du gaz carbonique et des hydrocarbures des cordiérites. Cinetique des mécanismes. Détermination des sites. Intérêt pétrogénétique. Bull Mineral 104:325–338
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Armbruster, T. Ar, N2, and CO2 in the structural cavities of cordierite, an optical and X-ray single-crystal study. Phys Chem Minerals 12, 233–245 (1985). https://doi.org/10.1007/BF00311293
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00311293