Abstract
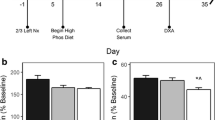
Predicting the course of parathormone (PTH)-elicited bone turnover in both humans and experimental rat models with moderate chronic uremia, using only standard clinical chemistry analyses, is often difficult. Consequently, rat bone from 1+2/3 nephrectomized animals, after 230 days of progressive renal failure, was examined for PTH-stimulated adenylate cyclase (AC) and phospholipase C (PLC) activities. Correlations to biological parameters related to the function of bone and kidney were made. Reduced renal function was demonstrated by increased serum creatinine; circulating 1,25 dihydroxyvitamin D3 below detection level; diminished renal PTH-elicited AC activity; and decreased urinary cAMP excretion. PTH-activated renal PL-C was also reduced. However, no significant differences were seen in urine creatinine, calcium, phosphate, and hydroxyproline, nor in serum PTH, alkaline phosphatase, calcium, and phosphate. Notwithstanding, renal osteodystrophy developed as estimated by increased plasticity of the long bones, as well as reduction of the diaphyseal (Dd) and inner femoral midshaft (Di) diameters. Femoral cancellous bone exhibited a substantial elevation of both eroded surface (ES) and osteoid surface (OS) as well as a marked reduction in trabecular bone volume (TBV). Calvarial PTH-activated AC was enhanced, whereas corresponding PL-C was markedly reduced. PTH-enhanced AC correlated positively with ES and negatively with Di, respectively. PTH-enhanced PL-C, however, correlated positively with bone calcium content and negatively with ES. Our results indicate that bone modeling and remodeling are to a large extent related to PTH-elicited signaling systems, and cannot easily be predicted by standard clinical chemistry analyses.
Similar content being viewed by others
References
Coburn JW, Henry DA (1984) Renal osteodystrophy. Adv Intern Med 30:387–424
Cushner HM, Adams ND (1986) Review: renal osteodystrophy—pathogenesis and treatment. Am J Med Sci 291:264–275
Mankin HJ (1990) Rickets, osteomalacia, and renal osteodystrophy—an update. Orthop Clin North Am 21(1):81–96
Potts JT Jr, Kronenberg HM, Rosenblatt M (1982) Parathyroid hormone: chemistry, biosynthesis, and mode of action. Adv Prot Chem 35:296–323
Klein KL, Maxwell MH (1984) Renal osteodystrophy. Orthop Clin North Am 15(4):687–695
Coburn JW, Slatopolsky E (1981) Vitamin D, parathyroid hormone, and renal osteodystrophy. In: Brenner B, Rector F (eds) The kidney. WB Saunders, Philadelphia, pp 2213–2205
Jablonski G, Klem KH, Attramadal A, Dahl E, Rønningen H, Gautvik KM, et al. (1993) Surgically induced uremia in rats I: effect on bone strength and metabolism. Biosci Rep 13(5):275–287
Jüppner H, Abou-Samra A-B, Freeman M, Kong XF, Schipani E, Richards J, et al. (1991) A G-protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science 254:1024–1026
Abou-Samra AB, Jüppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts JT Jr et al. (1992) Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular-free calcium. Proc Natl Acad Sci USA 89(7):2732–2736
Gordeladze JO, Gautvik KM (1986) Hydroxycholecalciferols modulate parathyroid hormone and calcitonin-sensitive adenylyl cyclase in bone and kidney of rats. A possible physiological role for 24,25-dihydroxy vitamin D3. Biochem Pharmacol 35(6): 899–902
Jackowski S, Rettenmier CW, Sherr CJ, Rock CO (1986) A guanine nucleotide-dependent phosphatidylinositol 4,5-diphosphate phospholipase C in cells transformed by the v-fms and v-fes oncogenes. J Biol Chem 261:4978–4985
Engesæter LB, Ekeland A, Langeland N (1978) Methods for testing the mechanical properties of the rat femur. Acta Orthop Scand 49:512–518
Dahl E, Nordal KP, Halse J, Attramadal A (1988) Histomorphometric analysis of normal bone from the iliac crest of Norwegian subjects. Bone Miner 3:369–377
Parfitt AM, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM (1987) Bone histomorphometry nomenclature, symbols and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Danielsen CC, Mosekilde Li, Andreassen TT (1992) Long-term effect of orchidectomy on cortical bone from rat femur: bone mass and mechanical properties. Calcif Tissue Int 50:169–174
Danielsen CC, Mosekilde Li, Svenstrup B (1993) Cortical bone mass, composition, and mechanical properties in female rats in relation to age, long-term ovariectomy, and estrogen substitution. Calcif Tissue Int 52:26–33
Oftebro H, Falch JA, Holmberg I, Haug E (1988) Validation of radioreceptor assay for 1,25-dihydroxyvitamin D using selected ion monitoring GC-MS. Clin Chim Acta 176:157–168
Gautvik KM, Teig V, Halvorsen JF, Arnesen E, Myhre L, Heimann P (1979) Development of sequence-specific radioimmunoassay of human parathyroid hormone and its use in the diagnosis of hyperparathyroidism. Scand J Clin Lab Invest 39:469–478
Gordeladze JO, Halse J, Djøseland O, Haugen HN (1978) A simple procedure for determination of hydroxyproline in urine and bone. Biochem Med Metab Biol 20:23–30
Sancho JJ, Duh QY, Oms L, Sitges-Serra A, Hammond ME, Arnaud CD, Clarc OH (1989) A new experimental model for secondary hyperparathyroidism. Surgery 106(6):1002–1008
Epstein S (1988) Serum and urinary markers of bone remodeling: assessment of bone turnover. Endocr Rew 9(4):437–449
Posen S, Lee C, Vines R (1977) Transient hyperphosphatasemia of infancy—an insufficiently recognized syndrome. Clin Chem 23(2):292–294
McComb RB, Bowers GN Jr, Posen S (1979) Serum alkaline phosphatase in patients with skeletal disorders: general consideration. In: Alkaline phosphatase. Plenum Press, New York, pp 570–571
Malluche HH, Smith AJ, Abreo K, Faugere MC (1984) The use of deferoxamine in the management of aluminium accumulation in bone in patients with renal failure. N Engl J Med 311(3):140–144
Deacon AC, Humle P, Hesp R, Green JR, Tellez M, Reeve J (1987) Estimation of whole body bone resorption rate: a comparison of urinary hydroxyproline excretion with two radioisotopic tracer methods in osteoporosis. Clin Chim Acta 166:297–306
Halse J, Gordeladze JO (1978) Urinary hydroxyproline excretion in acromegaly. Acta Endocrinol 89:483–491
Kivirikko KI (1970) Urinary excretion of hydroxyproline in health and disease. Int Rev Connect Tissue Res 15:93–163
Delmas PD (1990) Biochemical markers of bone turnover for clinical assessment of metabolic bone disease. Endocrinol Metab Clin North Am 19:1–18
Jouishomme H, Whitffield JR, Chakravarthy B, Durkin JP, Gagnon L, Isaacs RJ (1992) The protein kinase-C activation domain of the parathyroid hormone. Endocrinology 130(1):53–60
Fujimori A, Cheng S-L, Avioli LV, Civitelli R (1992) Structure-function relationship of parathryoid hormone: activation of phospholipase-C, protein kinase-A and-C in osteosarcoma cells. Endocrinology 130(1):29–36
Seitz PK, Nickols GA, Nickols MA, McPherson MB, Cooper CW (1990) Radioiodinated rat parathyroid hormone-(1-34) binds to its receptor on rat osteosarcoma cells in a manner consistent with two classes of binding sites. J Bone Miner Res 5(4):353–359
Cole JA, Carnes DL, Forte LR, Eber S, Poelling RE, Thorne PK (1989) Structure-activity relationships of parathyroid hormone analogs in the opossum kidney cell line. J Bone Miner Res 4(5):723–730
Quamme G, Pfeilschifter J, Murer H (1989) Parathyroid hormone inhibition of Na+/phosphate cotransport in OK cells: generation of second messengers in the regulatory cascade. Biochem Biophys Res Commun 1588(3):951–957
Klem KH, Jablonski G, Sæther O, Jarosz G, Gautvik KM, Gordeladze JO (1990) 1,25-dihydroxyvitamin D-3 and 24,25-dihydroxyvitamin D-3 affect parathormone (PTH)-sensitive adenylate cyclase activity and alkaline phosphatase secretion of osteoblastic cells through different mechanisms of action. Biochim Biophys Acta 1054:304–310
Mortensen BM, Gordeladze JO, Aksnes L, Gautvik KM (1990) Long-term administration of Vitamin D3 metabolites alters PTH-responsive osteoblastic adenylate cyclase in rats. Calcif Tissue Int 46:339–345
Gordeladze JO, Mortensen B, Nordal K, Halse J, Dahl E, Aksnes L, et al. (1987) The effect of parathyroid hormone (PTH) and 24,25-dihydroxy-vitamin D3 on adenylyl cyclase of iliac crest biopsies: diagnostic and prognostic tool for evaluation and treatment of uremic patients. Scand J Clin Lab Invest (suppl) 47(186):13–20
Berstein G, Blank JL, Smrcka AV, Higashijima T, Sternweis PC, Exton JH et al. (1992) Reconstitution of agonist-stimulated phosphatidylinositol 4,5-bisphosphate hydrolysis using purified m1 muscarinic receptor, Gq/11, and phospholipase C-β1. J Biol Chem 267(12):8081–8088
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jablonski, G., Danielsen, C.C., Mosekilde, L. et al. Surgically induced uremia in rats II: Osseous PTH-susceptible signaling systems as predictors of bone resorption. Calcif Tissue Int 55, 281–287 (1994). https://doi.org/10.1007/BF00310407
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00310407