Summary
The integument of Oncopeltus fasciatus is made up of a vacuolated and a pigmented epidermal cell layer. This double layered integument is present from late embryo to adult in male and female animals reared on milkweed or sunflower seeds. Experiments with a labelled glycoside as well as retrograde ink injections suggest that O. fasciatus concentrates cardiac glycosides, normally derived from the host plants, within the vacuolated epidermal cell layer throughout its life cycle. In the adult, droplets of glycoside-rich fluid appear at precise points along the dorsolateral margins when external pressure is applied to the thorax and abdomen. This pressure causes separation of cuticular flanges in the metathoracic epimeral lobe and rupture of the cuticle in restricted areas in the mesothorax and abdomen. In addition the pigmented epidermal cell layer and the distal membranes of the vacuolated epidermal cell layer rupture with the result that the contents of the vacuolated cell layer are eliminated onto the surface of the animal where they are retained as discrete droplets by the cuticular morphology. Release of cardiac glycosides into the haemolymph is prevented by a thick basal lamina on the haemolymph side of the vacuolated epidermal cells. No specialized muscles involved with fluid release were observed. The vacuolated epidermal cells do not show ultrastructural features characteristic of actively transporting tissues, i.e., abundant mitochondria and elaborate membrane infoldings. This suggests that glycoside sequestration is essentially a passive process and should not be associated with any physiological cost. Large concentration gradients of cardiac glycosides are maintained across the basal lamina, basal plasma and vacuolar membranes of the vacuolated epidermal cell layer. Possible mechanisms by which O. fasciatus is able to concentrate cardiac glycosides as well as the possible function of this phenomenon are discussed.
Similar content being viewed by others
Abbreviations
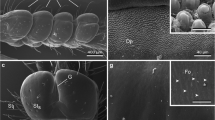
- A:
-
abdominal trabeculum
- Ap:
-
mesofurcal apodeme
- C:
-
metathoracic supracoxal lobe
- D:
-
metathoracic stink gland duct
- E:
-
metathoracic epimeral lobe
- Ep:
-
pigmented epidermal cell layer
- Ev:
-
vacuolated epidermal cell layer
- G:
-
last thoracic ganglion
- H:
-
haemocoel
- M:
-
midgut
- N:
-
nerve cord
- P:
-
second phragma
- R:
-
reproductive organ
- T:
-
trachea
- V:
-
dorsal vessel
- W:
-
wing
- bl:
-
basal lamina
- c:
-
cuticle
- cf:
-
cytoplasmic fragments
- cv:
-
coated vesicle
- d:
-
hemidesmosome
- ep:
-
epidermal cell
- en:
-
endocuticle
- ex:
-
exocuticle
- f:
-
flange
- fp:
-
foot processes
- gc:
-
glycoside compartment
- h:
-
hair
- is:
-
intersegmental region
- id:
-
ink deposits
- l:
-
lumen of metathoracic stink gland
- m:
-
mitochondria
- mb:
-
mushroom bodies
- mt:
-
microtrichia
- n:
-
nucleus
- p:
-
pigment granule
- s:
-
slit
- sp:
-
spine
- tsm:
-
tergosternal muscle
- v:
-
vacuole
References
Ashurst DE (1968) The connective tissues of insects. In: Smith RF, Mittler TE (eds) Annual review of entomology. Annual Reviews Inc Palo Alto 13:45–74
Baker PF, Willis JS (1972) Binding of the cardiac glycoside, ouabain to intact cells. J Physiol (London) 224:441–463
Brower LP (1969) Ecological chemistry. Sci Amer 220 (2):22–29
Brower LP, Brower JVZ (1964) Birds, butterflies, and plant poisons: a study in ecological chemistry. Zoologica 49:137–159
Brower LP, Glazier SC (1975) Localization of heart poisons in the monarch butterfly. Science 188:19–25
Calvert WH, Hedrick LE, Brower LP (1979) Mortality of the monarch butterfly (Danaus plexippus L.). Avian predation at five overwintering sites in Mexico. Science 204:4395–4397
Dorn A, Romer F (1976) Structure and function of prothoracic glands and oenocytes in embryos and last larval instars of Oncopeltus fasciatus (Insecta, Heteroptera). Cell Tissue Res 171:331–350
Duffey SS, Scudder GGE (1972) Cardiac glycosides in North American Asclepiadaceae, a basis for unpalatability in brightly coloured Hemiptera and Coleoptera. J Insect Physiol 18:63–78
Duffey SS, Scudder GGE (1974) Cardiac glycosides in Oncopeltus fasciatus (Dallas) (Hemiptera: Lygaeidae). I. The uptake and distribution of natural cardenolides in the body. Can J Zool 52:283–290
Duffey SS, Blum MS, Isman MB, Scudder GGE (1978) Cardiac glycosides: a physical system for their sequestration by the milkweed bug. J Insect Physiol 24:639–645
Govind CK, Dandy JWT (1970) The thoracic mechanism of the milkweed bug Oncopeltus fasciatus (Dallas) (Heteroptera: Lygaeidae). Can Entomol 102:1057–1074
Graham JDP, Staddon BW (1974) Pharmacological observations on body fluids from the milkweed bug Oncopeltus fasciatus (Dallas) (Heteroptera: Lygaeidae). J Entomol (A) 48:177–183
Isman MB (1977) Dietary influence of cardenolides on larval growth and development of the milkweed bug Oncopeltus fasciatus. J Insect Physiol 23:1183–1187
Isman MB, Duffey SS, Scudder GGE (1977) Cardenolide content of some leaf- and stem-feeding insects on temperate North American milkweeds (Asclepias spp.). Can J Zool 55:1024–1028
Johansson AS, Bräten T (1970) Cuticular morphology of the scent gland areas of some Heteropterans. Entomol Scand 1:258–262
Lawrence PA (1966) Development and determination of hairs and bristles in the milkweed bug Oncopeltus fasciatus (Lygaeidae, Hemiptera). J Cell Sci 1:475–498
Neville AC (1975) Biology of the arthropod cuticle. Springer, Berlin Heidelberg New York
Oschman JL, Berridge MJ (1971) The structural bais of fluid secretion. Fed Proc 30:49–56
Parsons JA (1965) A digitalis-like toxin in the monarch butterfly Danaus plexippus L. J Physiol (London) 178:290–304
Pasteels JM, Daloze D (1977) Cardiac glycosides in the defensive secretion of chrysomelid beetles: evidence for their production by the insects. Science 197:70–72
Roeske CN, Seiber JN, Brower LP, Moffitt CM (1976) Milkweed cardenolides and their comparative processing by monarch butterflies (Danaus plexippus L.). Rec Adv Phytochem 10:93–167
Rothschild M (1966) Experiments with captive predators and the poisonous grasshopper Poekilocerus bufonius. Proc Royal Ent Soc Lond (C) 31:32
Rothschild M (1973) Secondary plant substances and warning colouration in insects. In: van Emden HF (ed) Insect/Plant Relationships. Blackwell Scientific Publications Oxford, pp 59–83
Rothschild M, Kellett DN (1972) Reactions of various predators to insects storing heart poisons (cardiac glycosides) in their tissues. J Entomol (A) 46:103–110
Rothschild M, Reichstein T (1974) Some problems with the storage of cardiac glycosides by insects. In press
Rothschild M, Reichstein T, von Euw J, Alpin R, Harman RRM (1970) Toxic Lepidoptera. Toxicon 8:293–299
Rothschild M, von Euw J, Reichstein T (1973) Cardiac glycosides in the polka-dot moth Syntomeida epilais Walk. (Ctenuchidae: Lep.) with some observations on the toxic qualities of Amata (= Syntomis) phegea (L.). Proc Roy Soc Ser B 183:227–247
Scudder GGE, Duffey SS (1972) Cardiac glycosides in the Lygaeinae (Hemiptera: Lygaeidae). Can J Zool 50:35–42
Skou JC (1965) Enzymatic basis for active transport of Na+ and K+ across cell membrane. Physiol Rev 45:596–617
Strong L (1971) Intracellular ducts in the epidermis of the male desert locust. J Insect Physiol 17:1823–1831
von Euw J, Fishelson L, Parsons JA, Reichstein T, Rothschild M (1967) Cardenolides (heart poisons) in a grasshopper feeding on milkweeds. Nature (London) 214:35–39
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Scudder, G.G.E., Meredith, J. Morphological basis of cardiac glycoside sequestration by Oncopeltus fasciatus (Dallas) (Hemiptera: Lygaeidae). Zoomorphology 99, 87–101 (1982). https://doi.org/10.1007/BF00310302
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00310302