Summary
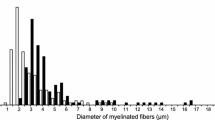
Morphometric observations have been made on the medial plantar division of the tibial nerve (MPD) and on the motor branches of the tibial nerve to the calf muscles (MBC) in rats ranging in age from weaning (3 weeks) to 12 months. Axon size, assessed by measurements of circumference and cross-sectional area, increased rapidly until 3 months with further slight increases between 3 and 9 months and a slight fall between 9 and 12 months. Axon size distributions were unimodal throughout in the MPD but bimodal for the MBC except at 3 weeks. Distributions of myelin thickness were bimodal throughout for both nerves. Scatter plots of g ratios (axon diameter: total fibre diameter) confirmed the presence of two fibre populations: a group of small fibres with relatively thin myelin sheaths, and a group of larger fibres within which sheath thickness was relatively less on the larger than on the smaller axons. These two fibres populations were less easily separable in the MBC than in the MPD nerves. These results document morphometrically the normal growth changes in the rat tibial nerve and also provide control data for the analysis of the effects of experimental procedures on the growth and maturation of peripheral nerve fibres.
Similar content being viewed by others
References
Arbuthnott ER, Boyd IA, Kalu KU (1980) Ultrastructural dimensions of myelinated peripheral nerve fibres in the cat. J Physiol 308:125–156
Bear RS, Schmitt FO (1937) Optical properties of axon sheaths of crustacean nerves. J Cell Comp Physiol 9:275–288
Beuche W, Friede RL (1985) A new approach toward analyzing peripheral nerve fibre populations. II. Foreshortening of regenerated internodes corresponds to reduced sheath thickness. J Neuropathol Exp Neurol 44:73–84
Beuche W, Friede RL (1986) Remodelling of nerve structure in experimental isoniazid neuropathy in the rat. Brain 109:759–769
Birren JE, Wall PD (1956) Age changes in conduction velocity, refractory period, number of fibres, connective tissue space and blood vessels of sciatic nerve of rats. J Comp Neurol 104:1–16
Bischoff A (1965) Problèmes et possibilités de l'exploration de l'ultra-structure du système nerveux périphérique. Rev Neurol (Paris) 112:337–384
Boughton TH (1906) The increase in the number and size of the medullated fibres in the oculomotor nerve of the white rat and of the cat at different ages. J Comp Neurol 16:153–165
Buchthal F, Rosenflack A (1966) Evoked action potentials and conduction velocity in human sensory nerves. Brain Res 3:1–119
Cox DR (1966) Notes on the analysis of mixed frequency distributions. Br J Math Stat Psychol 19:39–47
Dempster AP, Laird NM, Rubin D (1977) Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc [B] 39:1–38
Donaldson HH, Nagasaka G (1918) On the increase in the diameters of nerve cell bodies and fibres arising from them during the later stages of growth (albino rat). J Comp Neurol 29:529–552
Dunn EH (1912) The influence of age, sex, weight and relationship upon the number of medullated fibers and on the size if the largest fibers in the ventral root of the second cervical nerve of the albino rat. J Comp Neurol 22:131–157
Dyck PJ (1966) Histological measurements and fine structure of biopsied sural nerve: normal and in peroneal muscular atrophy, hypertrophic neuropathy and congenital sensory neuropathy. Mayo Clin Proc 41:742–774
Dyck PJ, Lambert EH, Nichols PC (1971) Quantitative measurements of sensation related to compound action potential and number and sizes of myelinated and unmyelinated fibers of sural nerve in health, Friedreich's ataxia, hereditary sensory neuropathy, and tabes dorsalis. In: Cobb WA (ed) Handbook of electroencephalography and clinical neurophysiology, vol 9. Elsevier, Amsterdam, pp 83–103
Evans DHL, Vizoso AD (1951) Observations on the mode of growth of motor fibers in rabbits during postnatal development. J Comp Neurol 95:429–461
Fraher JP (1972) A quantitative study of anterior root fibres during early myelination. J Anat 112:99–124
Fraher JP (1973) A quantitative study of anterior root fibres during early myelination. II. Longitudinal variation in myelin sheath thickness and axon circumference. J Anat 115:421–444
Fraher JP (1989) Axon-myelin relationships in rat cranial nerves III, IV and VI. A morphometric study of large and small fibre classes. J Comp Neurol (in press)
Fraher JP, Kaar GF (1985) The development of alpha and gamma motoneurone fibres in the rat. II. A comparative ultrastructural study of their central and peripheral myelination. J Anat 141:89–103
Friede RL, Beuche W (1985) A new approach toward analyzing peripheral nerve populations. I. Variance in sheath thickness corresponds to different geometric proportions of the internodes. J Neuropathol Exp Neurol 44:60–72
Friede RL, Beuche W (1985) Combined scatter diagrams of sheath thickness and fibre calibre in human nerves: changes with age and neuropathy. J Neurol Neurosurg Psychiatry 48:749–756
Friede RL, Bischhausen R (1982) How are sheath dimensions affected by axon caliber and internode length? Brain Res 235:335–350
Friede RL, Samorajski T (1967) Relation between the number of myelin lamellae and axon circumference in fibers of vagus and sciatic nerves of mice. J Comp Neurol 130:223–231
Friede RL, Brzoska J, Hartmann U (1985) Changes in myelin sheath thickness and internode geometry in the rabbit phrenic nerve during growth. J Anat 143:103–113
Jacobs JM, Love S (1985) Qualitative and quantitative morphology of the human sural nerve at different ages. Brain 108:897–924
Kaar GF, Fraher JP (1985) The development of alpha and gamma motoneuron fibres in the rat. I. A comparative ultrastructural study of their central and peripheral axon growth. J Anat 141:77–88
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137A-138A
King RHM, Craggs RI, Gross MLP, Tomkins C, Thomas PK (1983) Suppression of experimental allergic neuritis by cyclosporin-A. Acta Neuropathol (Berl) 59:262–268
Sanders FK (1948) Thickness of the myelin sheaths of normal and regenerating nerve fibres. Proc R Soc London, Ser B 135:323–357
Schröder JM, Bohl J, Brodda K (1978) Changes in the ratio between myelin thickness and axon diameter in the human developing sural nerve. Acta Neuropathol (Berl) 43:169–178
Schröder JM, Bohl J, Bardeleben U von (1988) Changes of the ratio between myelin thickness and axon diameter in human developing sural, femoral, ulnar, facial and cochlear nerves. Acta Neuropathol 76:471–483
Schwarzacher HG (1954) Markscheidendicke und Achsenzylinderdurchmesser in peripheren menschlichen Nerven. Acta Anat 21:26–46
Sharma AK, Thomas PK (1974) Peripheral nerve structure and function in experimental diabetes. J Neurol Sci 23:1–15
Sharma AK, Bajada S, Thomas PK (1981) Influence of streptozotocin-induced diabetes on myelinated nerve fibre maturation and on body growth in the rat. Acta Neuropathol (Berl) 53:257–265
Spencer PS, Schaumburg HH (1978) Pathobiology of neurotoxic axonal degeneration. In: Waxman S (ed) Physiology and pathobiology of axons. Raven Press, New York, pp 265–282
Sunderland S, Roche A (1958) Axon-myelin relationship in peripheral nerve fibres. Acta Anat 33:1–37
Thomas PK, Fraher JP, O'Leary D, Moran MA, Cole M, King RHM (1990) Relative growth and maturation of axon size and myelin thickness in the tibial nerve of the rat. 2. Effect of streptozotocin-induced diabetes. Acta Neuropathol 79:375–386
Williams PL, Wendell-Smith (1971) Some additional parametric variations between peripheral nerve fibre populations. J Anat 109:505–526
Author information
Authors and Affiliations
Additional information
Supported by an EEC Twinning Grant and by the Nuffield Foundation
Rights and permissions
About this article
Cite this article
Fraher, J.P., O'Leary, D., Moran, M.A. et al. Relative growth and maturation of axon size and myelin thickness in the tibial nerve of the rat. Acta Neuropathol 79, 364–374 (1990). https://doi.org/10.1007/BF00308712
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00308712