Abstract
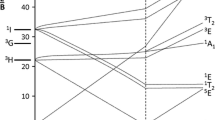
A comparative study of blue and green beryl crystals (from the region of Governador Valadares, Minas Gerais, Brazil) using electron paramagnetic resonance (EPR) and optical absorption (OA) spectroscopy is reported. The EPR spectra show that Fe3+ in blue beryl occupies a substitutional Al3+ site and in green beryl is localized in the structural channels between two O6 planes. On the other hand the infrared spectra show that the alkali content in the blue beryl is mostly at substitutional and/or interstitial sites and in green beryl is mostly in the structural channels. The OA spectra show two types of Fe2+. Thermal treatments above 200° C in green beryl cause the reduction of Fe3+ into Fe2+ accompanied by a change of color to blue. The blue beryl color does not change on heating. The kinetics of the thermal conversion of Fe3+ into Fe2+ is composed of two first order processes; the first one has an activation energy ΔE 1=0.30 eV and the second one has an activation energy ΔE 2=0.46 eV.
Similar content being viewed by others
References
Bakakin VV, Rylov GM, Belov NV (1967) Correlation between the chemical composition and unit cell parameters of beryl. Dokl Acad Sci USSR Earth Sci Sect 173:129–132
Bragg WL, West J (1926) The structure of beryl, Be3Al2Si6O18. Proc Roy Soc London Ser A 111:691–714
Dvir M, Low W (1960) Paramagnetic resonance and optical spectrum of iron in beryl. Phys Rev 119:1587–1591
Edgar A, Vance ER (1977) Electron paramagnetic resonance, optical absorption and magnetic circular dichroism studies of the CO− 3 molecular-ion in irradiated natural beryl. Phys Chem Minerals 1:165–178
Feklichev VG (1963) chemical composition of minerals of the beryl group. Geokhimiya 3:391–401
Goldman DS, Rossman GR, Parkin KM (1978) channel constituents in beryl. Phys Chem Minerals 3:225–235
Hawthorne FC, Cerny P (1977) The alkali-metal positions in Cs-Li beryl. Can Mineral 15:414–421
Parkin KM, Loeffler BM, Burns RG (1977) Mössbauer spectra of kyanite, aquamarine, and cordierite showing intervalence charge transfer. Phys Chem Minerals 1:301–311
Price DC, Vance ER, Smith G, Edgar A, Dickson BL (1976) Mössbauer effect studies of beryl. J Phys Colloq C6, Suppl 12, 37:811–817
Samoilovich MI, Tsinober LI, Dunin-Barkoviskii RL (1971) Nature of the coloring in iron — containing beryl. Sov Phys Crystallogr 16:147–150
Wood DL, Nassau K (1967) Infrared spectra of foreign molecules in beryl. J Chem Phys 47:2220–2228
Wood DL, Nassau K (1968) The characterization of beryl and emerald by visible and infrared absorption spectroscopy. Am Mineral 53:777–800
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blak, A.R., Isotani, S. & Watanabe, S. Optical absorption and electron spin resonance in blue and green natural beryl. Phys Chem Minerals 8, 161–166 (1982). https://doi.org/10.1007/BF00308238
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00308238