Summary
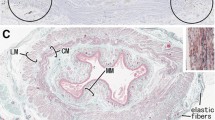
The longitudinal musculature (taeniae) of the rabbit proximal colon was studied by phase contrast and electron microscopy. The taenia coli of the rabbit has several structural features which are not found in other visceral muscles of this or other species, and which, on the other hand, are common in certain vascular muscles. Among the characteristics of taenia coli are: highly corrugated muscle cell profiles (even when the muscle is fixed in a resting condition), very high surface-to-volume ratio (1 μm2/0.5 μm3), large percentage volume of the extracellular space (about 40%), absence of intramuscular septa, and very large number of elastic fibres, which run mainly longitudinally in broad mesh-works parallel to the serosal surface. Several points of contact between elastic fibres and muscle cell membrane occur at all levels along the length of the cells, often involving 11-nm microfibrils; these contacts, and others involving collagen fibrils, are regarded as cell-to-stroma junctions. The taenia coli is virtually devoid of intramuscular blood vessels, including capillaries. The possibility that the lack of capillaries is related to the abundance of elastic material is discussed.
Similar content being viewed by others
References
Bülbring E, Tomita T (1969) Increase in membrane conductance by adrenaline in smooth muscle of the guinea-pig taenia coli. Proc R Soc B 172:89–102
Bülbring E, Brading AF, Jones AW, Tomita T (Eds) (1981) Smooth muscle: An assessment of current knowledge. Arnold: London
Clark JM, Glagov S (1979) Structural integration of the arterial wall. I. Relationships and attachments of medial smooth muscle cells in normally distended and hyperdistended aortas. Circ Res 40:587–602
Devine CE, Rayns DG (1975) Freeze-fracture studies of membrane systems in vertebrate muscles. II. Smooth muscle. J Ultrastruct Res 51:293–306
Devine CE, Somlyo AV, Somlyo AP (1972) Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol 52:690–718
Dingemans KP, Jansen N, Becker AE (1981) Ultrastructure of the normal human aortic media. Virchows Arch (Pathol Anat) 392:199–216
Drenckhahn D, Jeikowski H (1978) The myotendinous junction of smooth feather muscles (mm. pennati). Cell Tissue Res 194:151–162
Ehrlein HJ, Reich H, Schwinger M (1982) Physiological significance of contractions of the rabbit proximal colon. Q J Exp Physiol 67:407–417
Gabella G (1976) Quantitative morphological study of smooth muscle cells of the guinea-pig taenia coli. Cell Tissue Res 170:161–186
Gabella G (1979) Smooth muscle cell junctions and structural aspects of contraction. Br Med Bull 35:213–218
Gordon AR, Siegman MJ (1971a) Mechanical properties of smooth muscle. I. Length-tension and force velocity relations. Am J Physiol 221:1243–1249
Gordon AR, Siegman MJ (1971b) Mechanical properties of smooth muscle. II. Active state. Am J Physiol 221:1250–1254
Holstein AF, Orlandini GE, Baumgarten HG (1974) Morphological analysis of tissue components in the tunica dartos of man. Cell Tissue Res 154:329–344
Hörnicke H (1977) Some characteristics of the digestive physiology of the rabbit. (quoted by Snipes et al. 1982)
Komuro T (1982) The interstitial cells in the colon of the rabbit. Cell Tissue Res 222:41–51
Komuro T, Baluk P, Burnstock G (1982) An ultrastructural study of neurons and non-neuronal cells in the myenteric plexus of the rabbit colon. Neuroscience 7:1797–1806
Lowy J, Mulvany MJ (1973) Mechanical properties of guinea pig taenia coli muscles. Acta Physiol Scand 88:123–136
Mashima H, Yoshida T (1965) Effect of length on the development of tension in guinea-pig's taenia coli. Jpn J Physiol 15:463–477
Mitchell PC (1905) On the intestinal tract of mammals. Trans Zool Soc (London) 17:437–536
Ruckebusch Y, Fioramonti J (1976) The fusus coli of the rabbit as a pacemaker area. Experientia 32:1023–1024
Snipes RL (1978) Anatomy of the rabbit cecum. Anat Embryol 155:57–80
Snipes RL, Clauss W, Weber A, Hörnicke H (1982) Structural and functional differences in various divisions of the rabbit colon. Cell Tissue Res 225:331–346
Stephens NL, Chiu BS (1970) Mechanical properties of tracheal smooth muscle and effects of O2, CO2, and pH. Am J Physiol 219:1001–1008
Wolinsky H, Glagov S (1967) Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ Res 20:409–421
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gabella, G. The taenia of the rabbit colon, an elastic visceral muscle. Anat Embryol 167, 39–51 (1983). https://doi.org/10.1007/BF00304599
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00304599