Abstract
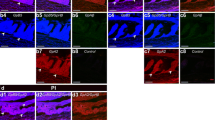
Immunohistochemical localization of lutropin β (LHβ) and follitropin β (FSHβ) in the pituitary gland of the frog Rana japonica was studied by the peroxidase-anti-peroxidase method and the two-face, double-labeling method with different-sized gold particles at the light-and electron-microscopic levels, respectively, using monoclonal antibodies against bullfrog LHβ and FSHβ. Light-microscopic immunohistochemistry indicated that approximately 66.0% of all the gonadotrophs in the pituitary contained both LHβ and FSHβ, whereas 33.4% of gonadotrophs contained only LHβ, and 0.6% contained only FSHβ. The staining intensity of LHβ and FSHβ varied from cell to cell. The gonadotrophs were classified into four types (Types I–IV) in terms of their ultrastructural and immunolabeling characteristics. Moreover, several secretory granule types were recognized according to differences in their shape and electron density. In all the cell types, both LHβ and FSHβ were often seen in the same secretory granules, but the proportion of granules bearing both hormones ranged from 5.5% in Type I to 32.7% in Type IV. Most secretory granules in Types I and II were immunolabeled with LHβ alone, whereas a small number of granules were immunolabeled with FSHβ alone. More immunolabeled FSHβ granules were present in Types III and IV than in Types I and II.
Similar content being viewed by others
References
Batten TC, Hopkins CR (1978) Discrimination of LH, FSH, TSH and ACTH in dissociated porcine anterior pituitary cells by light and electron microscope immunocytochemistry. Cell Tissue Res 192:107–120
Bendayan M (1982) Double immunocytochemical labeling applying the protein A-gold technique. J Histochem Cytochem 30:81–85
Bugnon C, Fellman D, Lenys D, Bloch B (1977) Etude cytoimmunologique des cellules gonadotropes et des cellules thyréotropes de l'adénohypophyse du rat. C R Soc Biol (Paris) 171:907–913
Child GV (1986) Functional ultrastructure of gonadotropes: a review. In: Paff D (ed) Currents topics in neuroendocrinology, vol 7. Springer, Berlin Heidelberg New York, pp 49–97
Child GV, Ellison DG, Garner LL (1980) An immunocytochemist's view of gonadotropin storage in the adult male rat: cytochemical and morphological heterogeneity in serially sectioned gonadotropes. Am J Anat 158:397–409
Dacheux F (1978) Ultrastructural localization of gonadotrophic hormones in the porcine pituitary using the immunoperoxidase technique. Cell Tissue Res 191:219–232
Dacheux F (1984) Subcellular localization of gonadotropic hormones in pituitary cells of the castrated pig with the use of pre-and post-embedding immunocytochemical methods. Cell Tissue Res 236:153–160
Dada MO, Campbell GT, Blake CA (1983) A quantitative immunocytochemical study of the luteinizing hormone and follicle-stimulating hormone cells in the adenohypophysis of adult male rats and adult female rats throughout the estrous cycle. Endocrinology 113:970–984
Doerr-Schott J (1964) Localisation au microscope électronique de l'activité phosphatasique acid dans les cellules β de l'hypophyse de la grenouille rousse Rana temporaria. C R Acad Sci III 258:1621–1623
Doerr-Schott J (1970) Répartition de l'activité phosphatasique acid dans les cellules gonadotropes de la grenouille au cours de leur cycle sécrétoire. Histochemie 23:21–35
Doerr-Schott J (1974) Localisation submicroscopique par cyto-immunoenzymologie de differents principes hormonaux de l'hypophyse de Rana temporaria L. J Microsc 20:151–164
Doerr-Schott J (1976) Immunohistochemistry of the adenohypophysis of non-mammalian vertebrates. Acta Histochem [Suppl] 22:S185-S223
Girod C, Dubois MP, Trouillas J (1980) Mise en évidence des cellules gonadotropes de l'adénohypophyse (pars distalis et pars tuberalis) du singe Macacus irus. Etude en immunofluorescence à l'aide d'anticorps anti-β-FSH humaine et anti-β-LH ovine. C R Soc Biol (Paris) 174:304–313
Girod C, Dubois MP, Trouillas J (1981) Immunohistochemical localization of FSH and LH in the pars distalis of vervet (Cercopithecus aethiops) and babbon (Papio harnadryas) pituitaries. Cell Tissue Res 217:245–257
Gracia-Navarro F, Doerr-Schott J (1982) Immunohistochemical detection of adenohypophyseal cells containing hormones in Rana ridibunda. Cell Tissue Res 222:687–690
Gracia-Navarro F, Licht P (1987) Subcellular localization of gonadotropic hormone LH and FSH in frog adenohypophysis using double-staining immunocytochemistry. Cell Tissue Res 35:763–769
Hanaoka Y, Hayashi H, Takahashi H (1984) Isolation and characterization of bullfrog gonadotropins. Gunma Symp Endocrinol 21:63–77
Hayashi H, Hayashi T, Hanaoka Y (1992) Amphibian lutropin and follitropin from the bullfrog Rana catesbeiana. Complete amino acid sequence of the alpha subunit. Eur J Biochem 203:185–191
Hayashi T, Hanaoka Y, Hayashi H (1993) Simple N-linked sugar chains are bound to the lutropin of the bullfrog Rana catesbeiana. Gen Comp Endocrinol 90:282–289
Herbert DC (1975) Localization of antisera to LHβ and FSHβ in the rat pituitary gland. Am J Anat 194:379–385
Herbert DC (1976) Immunocytochemical evidence that luteinizing hormone (LH) and follicle stimulating hormone (FSH) are present in the same cell type in the rhesus monkey pituitary gland. Endocrinology 98:1554–1557
Inoue K, Kurosumi K (1984) Ultrastructural immunocytochemical localization of LH and FSH in the pituitary of the untreated male rat. Cell Tissue Res 235:77–83
Kitajima K, Okada K, Kawaoi A (1980) Immunohistochemical localization of FSH and LH in the human pituitary glands. Acta Histochem Cytochem 13:157–163
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Mirecka J, Pearse AGE (1971) Localization of FSH and LH-producing cells in the pig adenohypophysis by an immunohistochemical technique. Folia Histochem Cytochem 9:365–371
Moriarty GC (1976) Immunohistochemistry of the pituitary glycoprotein hormones. J Histochem Cytochem 24:846–863
Moriarty GC, Garner LL (1977) Immunocytochemical studies of cells in the rat adenohypophysis containing both ACTH and FSH. Nature 265:356–358
Nakane PK (1970) Classification of anterior pituitary cell types with immunoenzyme histochemistry. J Histochem Cytochem 18:9–20
Park MK, Wakabayashi K (1986) Preparation of a monoclonal antibody to common amino acid sequences of LHRH and its application. Endocrinol Jpn 33:257–272
Park MK, Tanaka S, Hayashi H, Hanaoka Y, Wakabayashi K, Kurosumi K (1987) Production and characterization of a monoclonal antibody against the β-subunit of bullfrog lutropin. Gen Comp Endocrinol 68:82–90
Pelletier G, Leclerc R, Labrie F (1976) Identification of gonadotropic cells in the human pituitary by immunoperoxidase technique. Mol Cell Endocrinol 6:123–128
Phifer RF, Midgley AR, Spicer SS (1973) Immunohistologic and histologic evidence that follicle-stimulating hormone and luteinizing hormone are present in the same cell type in the human pars distalis. J Clin Endocrinol 36:125–141
Pierce JG, Parsons TF (1981) Glycoprotein hormones: structure and function. Ann Rev Biochem 50:465–495
Takahashi H, Hanaoka Y (1981) Isolation and characterization of multiple components of basic gonadotropin from bullfrog (Rana catesbeiana) pituitary gland. J Biochem 90:1333–1340
Tanaka S, Hanaoka Y, Wakabayashi K (1983) A homologous radioimmunoassay for bullfrog basic gonadotropin. Endocrinol Jpn 30:71–78
Tanaka S, Park MK, Takikawa H, Wakabayashi K (1985) Comparative studies on the electric nature of amphibian gonadotropin. Gen Comp Endocrinol 59:110–119
Tanaka S, Park MK, Hayashi H, Hanaoka Y, Wakabayashi K, Kurosumi K (1990) Immunocytochemical localization of the subunits of glycoprotein hormones (LH, FSH, and TSH) in the bullfrog pituitary gland using monoclonal antibodies and polyclonal antiserum. Gen Comp Endocrinol 77:88–97
Tanaka S, Sakai M, Park MK, Kurosumi K (1991) Differential appearance of the subunits of glycoprotein hormones (LH, FSH, and TSH) in the pituitary of bullfrog (Rana catesbeiana) larvae during metamorphosis. Gen Comp Endocrinol 84:318–327
Tixier-Vidal A, Tougard C, Kerdelhue B, Jutisz M (1975) Light and electron microscopic studies on immunocytochemical localization of gonadotropic hormones in the rat pituitary gland with antisera against ovine FSH, LH, LHα, and LHβ. Ann NY Acad Sci 254:433–460
Tougard C, Tixier-Vidal A (1988) Lactotrophs and gonadotrophs. In: Knobil E, Neill J et al (eds) The physiology of reproduction. Raven Press, New York, pp 1305–1333
Tougard C, Kerdelhue B, Tixier-Vidal A, Jutisz M (1973) Light and electron microscope localization of binding sites of antibodies aganist ovine luteinizing hormone and its two subunits in rat adenohypophysis using peroxidase-labeled antibody technique. J Cell Biol 58:503–521
Tougard C, Picart R, Tixier-Vidal A (1980) Immunocytochemical localization of glycoprotein hormones in the rat anterior pituitary. A light and electron microscope study using antisera against rat β subunits: a comparison between preembedding and postembedding methods. J Histochem Cytochem 28:101–114
Wakabayashi K, Tanaka S (1988) Assessment of specificity of antiserum for immunohistochemistry. Acta Histochem Cytochem 21:221–229
Watanabe T, Uchiyama Y, Grube D (1991) Topology of chromogranin A and secretogranin II in the rat anterior pituitary: potential marker proteins for distinct secretory pathways in gonadotrophs. Histochemistry 96:285–293
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mizutani, F., Iwasawa, H. & Tanaka, S. A morphometric analysis of the subcellular distribution of LHβ and FSHβ in secretory granules in the pituitary gonadotrophs of the frog (Rana japonica). Cell Tissue Res 277, 417–426 (1994). https://doi.org/10.1007/BF00300214
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00300214