Summary
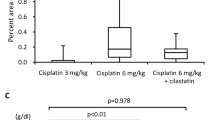
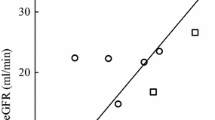
We compared the acute tubular nephrotoxicity of three platinum compounds in children and adults with solid tumors by monitoring the urinary excretion of alanine aminopeptidase, N-acetyl-β-D-glucosaminidase, and total protein. Cisplatin (100 mg/m2) was administered with mannitol, or at a twofold larger total dosage (50 mg/m2 per day for 4 days) in a 3% saline infusion. Carboplatin (300 mg/m2) was administered in combination with 5-fluorouracil, and iproplatin was administered in dosages ranging from 216 to 388 mg/m2. Enzymuria and proteinuria induced by cisplatin at a total dosage of 200 mg/m2 on a divided schedule did not significantly differ from that observed for the single 100 mg/m2 dose. Enzymuria and proteinuria induced by carboplatin and iproplatin were significantly less than that for cisplatin; however, one patient developed chronic tubular damage after three courses of carboplatin, and the acute tubular toxicity of iproplatin in one of 15 patients was exceptional. Our findings support the value of administering cisplatin in hypertonic saline on a divided schedule as a strategy to reduce acute tubular damage. Although carboplatin and iproplatin are less nephrotoxic than cisplatin, occasionally patients experience subclinical acute or chronic tubular damage that may lead to overt nephrotoxicity with continued therapy.
Similar content being viewed by others
References
Buamah PK, Howell A, Whitby H Harpur ES, Gescher A (1982) Assessment of renal function during high-dose cis-platinum therapy in patients with ovarian carcinoma. Cancer Chemother Pharmacol 8:281
Burstone MS, Folk JE (1956) Histochemical demonstration of aminopeptidase. J Histochem Cytochem 4:217
Canetta R, Rozencweig M, Carter SK (1985) Carboplatin: the clinical spectrum to date. Cancer Treat Rev 12 (suppl A):125
Carone FA, Ganote CE (1975) D-serine nephrotoxicity. The nature of proteinuria, glucosuria and aminoaciduria in acute tubular necrosis. Arch Pathol 99:658
Cohen AI, Harberg J, Citrin DL (1981) Measurement of urinary β2 in the detection of cisplatin nephrotoxicity. Cancer Treat Rep 65:1083
Corden BJ, Fine RL, Ozols RF, Collins JM (1985) Clinical pharmacology of high-dose cisplatin. Cancer Chemother Pharmacol 14:38
Creaven PJ, Madajewicz S, Pendyala L, Mittelman A, Pontes E, Spaulding M, Arbuck S, Soloman J (1983) Phase I clinical trial of cis-dichloro-trans-dihydroxy-bis-isoporopylamine platinum (IV) (CHIP). Cancer Treat Rep 67:795
Diener U, Knoll E, Langer B, Rautenstrauch H, Ratge D, Wisser H (1981) Urinary excretion of n-acetyl-β-D-glucosaminidase and alanine aminopeptidase in patients receiving amikacin or cis-platinum. Clin Chim Acta 112:149
Diener U, Knoll E, Ratge D (1982) Urinary excretion of alanine aminopeptidase and N-acetyl-β-glucosaminidase during sequential combination chemotherapy. J Clin Chem Clin Biochem 20:615
Forastiere AA, Wolf GT, Medvec BR, Baker SR (1985) Treatment of head and neck cancer with high dose cisplatin in hypertonic saline. Proc Am Soc Clin Oncol 4:14 (abstr)
Forastiere AA, Natale RB, Vogel WC, Kudla V, Takasugi BJ, Medvec B (1986) Carboplatin and 5-fluorouracil combination chemotherapy in advanced head and neck cancer. (Abstract) Proc Am Soc Clin Oncol 5:140
Goren MP, Wright RK, Horowitz ME (1986a) Cumulative renal tubular damage associated with cisplatin nephrotoxicity. Cancer Chemother Pharmacol 18:69
Goren MP, Wright RK, Osborne S (1986b) Two automated procedures for N-acetyl-β-D-glucosaminidase determination evaluated for detection of drug-induced tubular nephrotoxicity. Clin Chem 32:2052
Hayes DM, Cvitkovic E, Golbey RB, Scheiner E, Helson L, Krakoff IH (1977) High dose cis-platinum diammine dichloride. Amelioration of renal toxicity by mannitol diuresis. Cancer 39:1372
Jones BR, Bhalla RB, Mladek J, Kaleya RN, Gralla RJ, Alcock NW, Reidenberg MM (1980) Comparison of methods of evaluating nephrotoxicity of cis-platinum. Clin Pharmacol Ther 27:557
Jung K, Scholz D (1980) An optimized assay of alanine aminopeptidase activity in urine. Clin Chem 26:1251
Kuhn JA, Argy WP, Rakowski TA, Moriarty JK Jr, Shreiner GE, Schein PS (1980) Nephrotoxicity of cis-diammine-dichloro-platinum (II) as measured by urinary β-glucuronidase. Cancer Treat Rep 64:1083
Le Hir M, Dubach UC, Schmidt U (1979) Quantitative distribution of lysosomal hydrolases in the rat nephron. Histochemistry 63:245
Lelieveld P, van der Vijgh WJF, Veldhuizen RW, Van Velzen D, Van Putten LM, Atassi G, Danguy A (1984) Preclinical studies on toxicity, antitumour activity and pharmacokinetics of cisplatin and three recently developed derivatives. Eur J Cancer Clin Oncol 20:1087
Litterst CL (1981) Alterations in toxicity of cis-dichlorodiammineplatinum-II and in tissue localization of platinum as a function of NaCl concentration in the vehicle of aministration. Toxicol Appl Pharmacol 61:99
Madias NE, Harrington JT (1978) Platinum nephrotoxicity. Am J Med 65:307
Neter J, Wasserman W (1974) Applied linear statistical models. Richard D. Irvin, Homewood, Ill, p 146
Owens SE, Thatcher N, Sharma H, Adam N, Harrison R, Smith A, Zaki A, Baer JC, McAuliffe CA, Crowther D, Fox BW (1985) In vivo distribution studies of radioactively labelled platinum complexes; cis-dichlorodiammine platinum (II), cis-trans-dichlorodihydroxy-bis-(isopropylamine) platinum (IV), cis-dichloro-bis-cyclopropylamine platinum (II), and cis-diammine 1, 1-cyclobutanedicarboxylate platinum (II) in patients with malignant disease, using a gamma camera. Cancer Chemother Pharmacol 14:253
Ozols RF, Corden BJ, Jacob J, Wesley MN, Ostchega Y, Young RC (1984) High-dose cisplatin in hypertonic saline. Ann Intern Med 100:19
Ozols RF, Ostchega Y, Myers CE, Young RC (1985) Highdose cisplatin in hypertonic saline in refractory ovarian cancer. J Clin Oncol 3:1246
Pendyala L, Madajewicz S, Shashikant, BL, Arbuck SG, Creaven PJ (1985) Evaluation of the nephrotoxicity of iproplatin (CHIP) in comparison to cisplatin by the measurement of urinary enzymes. Cancer Chemother Pharmacol 15:203
Pfeifle CE, Howell SB, Felthouse RD Woliver TBS, Andrews PA, Markman M, Murphy MP (1985) High-dose cisplatin with sodium thiosulfate protection. J Clin Oncol 3:237
Pratt CB, Kamen BA, Winick N, Sartain P, Champion J, Ragab A (1986) Phase I study of cis-dichloro-trans-dihydroxy-bis (isopropylamine) platinum IV (iproplatin, CHIP) in pediatric patients. Proc Am Soc Clin Oncol 5:205 (abstr)
Stewart DJ, Mikhael NZ, Nanji AA, Nair RC, Kacew S, Howard K, Hirte W, Maroun JA (1985) Renal and hepatic concentrations of platinum: relationship to cisplatin time, dose and nephrotoxicity. J Clin Oncol 3:1251
Author information
Authors and Affiliations
Additional information
Supported in part by Biomedical Research Support grant RR05584 (MPG), Cancer Center Support (CORE) grant CA-21765, Childhood Solid Tumor Program Project grant CA-23099, American Lebanese Syrian Associated Charities and NCI N01-CM-07405
Rights and permissions
About this article
Cite this article
Goren, M.P., Forastiere, A.A., Wright, R.K. et al. Carboplatin (CBDCA), iproplatin (CHIP), and high dose cisplatin in hypertonic saline evaluated for tubular nephrotoxicity. Cancer Chemother. Pharmacol. 19, 57–60 (1987). https://doi.org/10.1007/BF00296257
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00296257