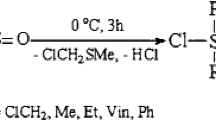Summary
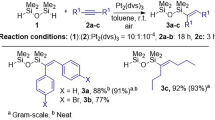
Hydro- and vinyl-bifunctionalized di- or tetrasiloxane were synthesized by degradative cleavage of functional cyclic siloxanes with methyllithium followed by quenching with a functional chlorosilane, or by ring-opening of hexamethylcyclotrisiloxane (D3) by functional alkyllithium followed by quenching with a functional chlorosilane. These bifunctionalized siloxanes were used as monomers in polyaddition with transition metal catalysts.
Similar content being viewed by others
References
Mori A, Hishida T, Soga Y, Kawakami Y (1995) Chem Lett 107 See also: Sieburth S M, Fresterbank L (1993) J Org Chem 58:6314 Sieburth S M, Mu W (1993) J Org Chem 58:7854
Mori A, Sato H, Mizuno K, Hiyama T, Shintani K, Kawakami Y (1996) Chem Lett in press.
Kawakami Y, Ajima K, Nomura M, Hishida T, Mori A in preparation.
Itsuno S, Chao D, Ito, K (1993) J Polym Sci Part A: Polym Chem 31:287 Shintani K, Soga Y, Mori A, Kawakami Y (1995) The Society of Polymer Science, Japan, Hokuriku Regional Meeting Preprint A-23
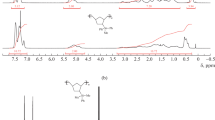
IR (neat) 2960, 2123, 1389, 1254, 1059, 909 cm−1; 1H NMR (CDCl3) δ 0.22 (d, J=2.8 Hz, 6H), 0.37 (s, 6H), 4.79 (heptet, J=2.7 Hz, 1H), 5.30 (d, J=10.9 Hz, 1H), 5.82 (d, J=17.6 Hz, 1H), 7.44 (d, J=8.0 Hz), 6.78 (dd, J=8.0, 17.6 Hz, 1H), 7.55 (d, J=8.0 Hz, 2H); 13C−{1H} NMR (CDCl3) δ 0.53, 0.83, 30.3, 114.3, 125.5, 133.2, 136.8, 138.4.
IR (neat) 2961, 2124, 1596, 1407, 1254, 1062, 910 cm−1; 1H NMR (CDCl3) δ 0.16 (s, 6H), 0.18 (d, J=2.8 Hz, 6H), 4.70 (heptet, J=2.8 Hz, 1H), 5.74 (dd, J=20.0, 4.2 Hz, 1H), 5.95 (dd, J=14.8, 4.1 Hz, 1H), 6.13 (dd, J=14.8, 20.0 Hz, 1H); 13C−{1H} NMR (CDCl3) δ 0.08, 0.83, 131.8, 139.1.
Paquette L A Ed(1995) Encyclopedia of Reagents for Organic Synthesis John Wiley & Sons, New York, 1:110
IR (neat) 3080, 2961, 2917, 2124, 1632, 1420, 1254, 1159, 1065, 908, 835, 767 cm−1; 1H-NMR (CDCl3) δ 0.10 (s, 6H), 0.17 (d, J=2.8 Hz, 6H), 1.57 (d, J=9.1 Hz, 2H,), 4.69 (hextet, J=2.8 Hz, 1H), 4.88 (dd, J=9.1, 16.8 Hz, 2H), 5.72–5.87 (m, 1H); 13C−{1H} NMR (CDCl3) δ 0.44, 0.83, 26.1, 113.4, 134.2.
Ojima I in Patai S, Rappoport Z Ed(1989) The Chemistry of Organic Silicon Compounds, John Wiley & Sons, New York Chap 25:1479
Chandra G, Lo P Y, Hitchcock P B, Lappert M F (1987) Organometallics 6: 191
Fleming I in Jones D N Ed(1979) Comprehensive Organic Chemistry, Pergamon, Oxford, 3:541
Dvornic, P R, Gerov V V, Govedarica M N (1994) Macromolecules 27: 7575
Tsumura M, Iwahara T, Hirose T (1995) Polym J 27: 1048
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shintani, K., Ooi, O., Mori, A. et al. A facile synthesis of hydro- and vinyl-functionalized di- and tetrasiloxanes and polyaddition via hydrosilylation. Polymer Bulletin 37, 705–710 (1996). https://doi.org/10.1007/BF00295766
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00295766