Abstract
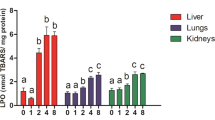
Male, weanling Blue-Spruce rats were treated with naphthalene (p.o.) in defined dose increments up to 750 mg/kg body weight over 9 weeks. At necropsy, treated rats showed a 20% decrease in body weight compared to controls. Naphthalene treatment resulted in enhanced peroxidation (p<0.001) only in the liver. This increased peroxidation was associated with reductions (p<0.05) in the activity of the selenoenzyme glutathione peroxidase in hepatic cytosolic fractions and an associated increase (p<0.05) in the selenium-independent glutathione peroxidase. No increase in peroxidation was observed in the lung, eye or heart of these rats and the activities of the selenoenzyme and the selenium-independent glutathione peroxidases were also unaffected by naphthalene in these organs. Naphthalene also did not affect superoxide dismutase activity in any of the organs examined. Thus, in addition to the known effects of naphthalene on tissue glutathione, naphthalene-induced reductions in the selenoenzyme glutathione peroxidase can also contribute to peroxidation in the liver and must be considered as a contributing factor in naphthalene toxicity in vivo.
Similar content being viewed by others
References
Anziulewicz BH, Dick HJ, Chiarulli EE (1959) Transplacental naphthalene poisoning. Am J Obstet Gynecol 78: 518–521
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72: 248–254
Burk RF, Lawrence RA, Lane JM (1980) Liver necrosis and lipid peroxidation in the rat as a result of paraquat and diaquat administration: effect of selenium deficiency. J Clin Invest 65: 1024–1031
Ghetti G, Mariani L (1956) Alterazioni ocularida naftalina. Med Lavoro 47: 533–538
Heyningen VR (1967) The metabolism of naphthalene and its toxic effect on the eye. Biochem J 102: 842–852
Jamall IS (1987) Differential effects of cadmium on cytosolic and mitochondrial glutathione levels in the rat heart. FEBS Lett 214: 62–64
Jamall IS, Smith JC (1985a) Effects of cadmium on glutathione peroxidase, superoxide dismutase and lipid peroxidation in the rat heart: a possible mechanism of cadmium cardiotoxicity. Toxicol Appl Pharmacol 80: 33–42
Jamall IS, Smith JC (1985b) Effect of cadmium treatment on glutathione peroxidase activity and lipid peroxidation in kidney and liver of rats maintained on various levels of dietary selenium. Arch Toxicol 58: 102–105
Jamall IS, Sprowls JJ (1987) Effects of cadmium and dietary selenium on cytoplasmic and mitochondrial antioxidant defense systems in the heart of rats fed high dietary copper. Toxicol Appl Pharmacol 87: 102–110
Jerina DM, Daly JW, Witkop B, Zaltzman-Nirenberg, Udenfriend S (1970) Naphthalene oxide as an intermediate in the microsomal hydroxylation of naphthalene. Biochemisry 9: 147–157
Kako KJ (1987) Free radical effects on membrane protein in myocardial ischemia/reperfusion injury. J Mol Cell Cardiol 19: 209–211
Kappus H (1987) Oxidative stress in chemical toxicity. Arch Toxicol 60: 144–149
Koch HR, Doldi K, Hockwin O (1976) Naphthalene cataract development. Doc Ophthal 3: 323–332
Lawrence RA, Burk RF (1978) Species, tissues and subcellular distribution of non-selenium glutathione peroxidase activity. J Nutr 108: 211–215
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70: 158–169
Pritsos CA, Pardini RS (1984) A redox cycling mechanism of action for 2,3-dichloro-1,4-naphthoquinone with mitochondrial membranes and the role of sulfhydryl groups. Biochem Pharmacol 33: 3771–3777
Rao GN, Sadasivudu B, Collier E (1983) Studies on glutathione S-transferase, glutathione peroxidase and glutathione reductase in human normal and cataractous lenses. Ophthalmic Res 15: 173–179
Reiter R, Burk RF (1987) Effect of oxygen tension on the generation of alkanes and malondialdehyde by peroxidizing rat liver microsomes. Biochem Pharmacol 36: 925–929
Schlafer M, Shephard BM (1984) A method to reduce interference by sucrose in the detection of thiobarbituric acid-reactive substances. Anal Biochem 137: 269–276
Schramm H, Robertson LW, Oesch F (1985) Differential regulation of hepatic glutathione-S-transferase and glutathione peroxidase activities in the rat. Biochem Pharmacol 34: 3735–3739
Sevanian A, Hochstein P (1985) Mechanisms and consequences of lipid peroxidation in biological systems. Annu Rev Nutr 5: 365–390
Smith MT, Evans CG (1984) Inhibitory effect of superoxide-generating quinones on superoxide dismutase. Biochem Pharmacol 33: 3109–3110
Srivastava SK, Lal AK, Ansari NH (1980) Defense system of the lens against oxidative damage: Effect of oxidative challenge on cataract formation on glutathione peroxidase deficient acatalasemic mice. Exp Eye Res 31: 425–433
Tan KH, Meyer DJ, Belin J, Ketterer B (1984) Inhibition of microsomal lipid peroxidation by GSH and GSH-S-transferases B and AA. Biochem J 220: 243–252
Walseth F, Toftgard R, Nilsen OG (1982) Phthalate esters. I. Effects on cytochrome P-450 mediated metabolism in rat liver and lung, serum erythrocyte activities and serum protein levels. Arch Toxicol 50: 1–10
Warren DL, Brown D, Buckpitt AR (1982) Evidence for cytochrome P-450 mediated metabolism in the bronciolar damage by naphthalene. Chem Biol Inter 40: 287–303
Winterbourn CC, Hawkins RE, Brian M, Carrell RW (1975) The estimation of red cell superoxide dismutase activity. J Lab Clin Med 85: 337–341
Author information
Authors and Affiliations
Additional information
Supported, in part, by NIH Grant ES 03370
Rights and permissions
About this article
Cite this article
Germansky, M., Jamall, I.S. Organ-specific effects of naphthalene on tissue peroxidation, glutathione peroxidases and superoxide dismutase in the rat. Arch Toxicol 61, 480–483 (1988). https://doi.org/10.1007/BF00293694
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00293694