Abstract
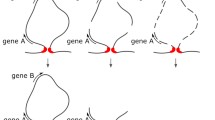
Short DNA regions, known to contain replication origins, were isolated from 2 M NaCl resistant nuclear structures of Physarum polycephalum after predigestion with DNase. Regions of 100 bp average length were cloned and sequenced. About 25% of the clones contained direct repeats of 12 to 16 bp and variable base sequences, that have been shown to possess the potential of playing a crucial role in the control of DNA replication. In one of the two alternative three-dimensional configurations such repeats expose single-stranded loops that can function as sites for the initiation of new DNA strands. As these regions are converted into full-length duplexes by their own replication, reinitiaton at the same site is excluded. Restoration of the initiable configuration is considered to be coupled to structural rearrangements involved in the transient condensation of chromosomes in mitosis. This mechanisms ensures that any part of the entire eukaryotic genome is reproduced just a single time during one cell cycle.
Similar content being viewed by others
References
Aelen JMA, Opstelten RJG, Wanka F (1983) Organization of DNA replication in Physarum. Attachment of origins of replicons and replication forks to the nuclear matrix. Nucleic Acids Res 11:1181–1196
Beach D, Piper M, Shall S (1980) Isolation of newly-initiated DNA from the early S phase of the synchronous eukarote Physarum polycephalum. Exp Cell Res 129:211–221
Bekers AGM, Gijzen HJ, Taalman RDFM, Wanka F (1981) Ultrastructure of the nuclear matrix from Physarum polycephalum during the mitotic cycle. J Ultrastruct Res 75:352–362
Bekers AGM, Pieck ACM, Rijken AAM, Wanka F (1986) Evidence for the persistence of a decondensed chromosome scaffold in the interphase nucleus. J Cell Sci 86:155–171
Berezney R (1984) Organization and functions of the nuclear matrix. In: Hnilica NS (ed) Chromosomal nonhistone proteins, 4. CRC Press, Boca Raton, pp 119–180
Blow JJ, Laskey RA (1988) A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature 332:546–548
Blumenthal AB, Kriegstein HJ, Hogness DS (1974) The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harbor Symp Quant Biol 38:205–223
Bodnar JW (1988) A domain model for eukaryotic DNA organization. A molecular basis for cell differentiation and chromosome evolution. J Theor Biol 132:479–507
Bramhill D, Kornberg A (1988a) Duplex opening by DNA A protein at novel sequences in initiation of replication at the origin of E. Coli chromosomes. Cell 52:743–755
Bramhill D, Kornberg A (1988b) A model for initiation of DNA replications. Cell 54:915–918
Britten RJ, Graham DJ, Neufeld BR (1974) Analysis of repeating DNA sequences by reassociation. Methods Enzymol 29:363–418
Carri MT, Micheli G, Graziano E, Pace T, Buongiorno-Nardelli M (1986) The relationship between chromosomal origins of replication and the nuclear matrix during the cell cycle. Exp Cell Res 164:426–436
Daniel JW, Baldwin HH (1964) Methods of culture for plasmodial myxomycetes. Methods Cell Physiol 1:9–41
Dijkwel PA, Mullenders LHF, Wanka F (1979) Analysis of the attachment of replicating DNA to a nuclear matrix in mammalian interphase nuclei. Nucleic Acids Res 6:219–230
Dijkwel PA, Wenink PW, Poddighe J (1986) Permanent attachment of replication origins to the nuclear matrix in BHK cells. Nucleic Acids Res 14:3241–3249
Edenberg HJ, Huberman JA (1975) Eukaryotic DNA replication. Annu Rev Genet 9:245–284
Feldherr CM (1972) Structure and function of the nuclear envelope. Adv Cell Mol Biol 2:273–307
Fields AP, Shaper JW (1988) A major 62 Kd intranuclear matrix polypeptide is a component of metaphase chromosomes. J Cell Biol 107:833–840
Forney J, Henderson ER, Blackburn EH (1987) Identification of the acellular slime molds Didymium irridis and Physarum polycephalum. Nucleic Acids Res 15:9143–9151
Gasser SM, Laemmli UK (1986) Cohabitation of scaffold binding regions with upstream/enhancer elements of three developmentally regulated genes of D. melanogaster. Cell 46:521–530
Güttes E, Güttes S (1964) Mitotic synchrony in the plasmodia of Physarum polycephalum and mitotic synchronization by coalescence of microplasmodia. Methods Cell Physiol 1:43–53
Güttes E, Güttes S (1968) Regulation of DNA replication in the nuclei of the slime mold Physarum polycephalum. Transplantation of nuclei by plasmodial coalescence. J Cell Biol 37:258–291
Hameister H, Sperling K (1984) Description of a chromosome replication unit in individual prematurely condensed S-phase chromosomes. Cromosoma 90:389–393
Handeli S, Klar A, Meuth M, Cedar H (1989) Mapping replication units in animal cells. Cell 57:909–920
Hanks SK, Rao PN (1980) Initiation of DNA synthesis in the prematurely condensed chromosomes of G1 cells. J Cell Biol 87:258–291
Hardman N, Jack PL, Fergie RC, Gerrie LM (1980) Sequence organization in nuclear DNA from Physarum polycephalum. Eur J Biochem 103:247–257
Huberman JA, Riggs AD (1968) On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol 32:327–341
Hunt BF, Vogelstein B (1981) Association of newly replicated DNA with the nuclear matrix of Physarum polycephalum. Nucleic Acids Res 9:349–363
Ito T, Sakaki Y (1987) Nuclear matrix associated regions of rat α2-macroglobulin gene. Biochem Biophys Res Commun 149:449–454
Joshua-Tor L, Rabinovich D, Hope H, Frolow F, Appella E, Sussman JL (1988). The three-dimensional structure of a DNA duplex containing looped-out bases. Nature 334:82–84
Karch F, Török I, Tissieres A (1981) Extensive regions of homology in front of the two hsp70 heat shock variant genes in Drosophila melanogaster. J Mol Biol 148:219–230
Kornberg A (1980) DNA synthesis. Freeman, San Francisco
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: A laboratory manual Cold Spring Harbor Laboratory, New York
Marsden MGF, Laemmli UK (1979) Metaphase chromosome structure: Evidence for a radial loop model. Cell 17:849–858
Meessing J, Vieira J (1982) A new pair of M13 vectors for selecting either strand of a double digest restriction fragment. Gene 19:269–276
Miller M, Harrison RW, Wlodawer A, Appella E, Sussman JL (1988) Crystal structure of a 15-mer DNA duplex containing unpaired bases. Nature 334:85–86
Mitchelson KR, Bekers AGM, Wanka F (1979) Isolation of a residual protein structure from nuclei of the myxomycete Physarum polycephalum. J Cell Sci 39:247–256
Mohberg J, Rusch HP (1977) Isolation and DNA content of nuclei of Physarum polycephalum. Exp Cell Res 66:305–316
Nelson WG, Pienta KG, Barak ER, Coffey DS (1986) The role of the nuclear matrix in the organization and function of DNA. Annu Rev Biophys Biophys Chem 15:457–475
Opstelten RJG (1987) Structural components of the cell nucleus and the organization of chromosomal DNA. Dissertation. Nijmegen University
Pieck ACM, van der Velden HMW, Rijken AM, Wanka F (1985) Protein composition of the chromosomal scaffold and interphase nuclear matrix. Chromosoma 91:137–144
Pieck ACM, Rijken AM, Wanka F (1987) Nuclear matrix and chromosomal scaffold peparations of in vitro cultured bovine liver cells have two proteins in common. FEBS Lett 212:276–280
Rao NP, Johnson RT (1970) Mammalian cell fusion: Studies on the regulation of DNA synthesis and mitosis. Nature 225:159–164
Razin SW, Kekelidze MG, Lukanidin EM, Scherrer K, Georgiev GP (1986) Replication origins are attached to the nuclear skeleton. Nucleic Acids Res 14:8189–8207
Rigby PJW, Dieckman M, Rhode C, Berg P (1977) Labeling deoxyribonuceic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol 113:237–251
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Van der Velden HMW, Wanka F (1987) The nuclear matrix — its role in the spatial organization an replication of eukaryotic DNA. Mol Biol Rep 12:69–77
Van der Velden HMW, van Willigen G, Wetzels RHW, Wanka F (1984) Attachment of origins of replication to the nuclear matrix and chromosomal scaffold. FEBS Lett 171:13–16
Waitz W, Loidl P (1988) In situ preparation of the nuclear matrix of Physarum polycephalum: Ultrastructural and biochemical analysis of different matrix isolation procedures. J Cell Sci 90:621–628
Wanka F, Mullenders LHF, Bekers AGM, Pennings LJ, Aelen JMA, Eygensteyn J (1977) Association of nuclear DNA with a rapidly sedimenting structure. Biochem Biophys Res Commun 74:739–747
Wanka F, Pieck ACM, Bekers AGM, Mullenders LHF (1982) The attachment of replicating DNA to the nuclear matrix. In: Maul GG (ed) the nuclear envelope and the nuclear matrix. Alan R. Liss, New York
Yanishevski RM, Prescott DM (1978) Late S phase cells (Chinese hamster ovary) induce early S phase labelling patterns in G1 nuclei. Proc Natl Acad Sci USA 75:3307–3311
Zannis-Hadjopoulos M, Kaufman G, Martin RG (1984) Mammalian DNA enriched for replication origins is enriched for snapback sequences. J Mol Biol 179:577–586
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Opstelten, R.J.G., Clement, J.M.E. & Wanka, F. Direct repeats at nuclear matrix-associated DNA regions and their putative control function in the replicating eukaryotic genome. Chromosoma 98, 422–427 (1989). https://doi.org/10.1007/BF00292787
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00292787