Summary
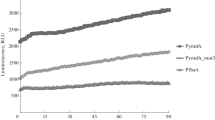
The nucleotide sequence of the 1uxA and luxB genes encoding the αβ heterodimeric luciferase from thermotolerant Vibrio harveyi CTP5 was determined. The DNA sequence of the CTP5 luxA and luxB genes is identical to the DNA sequence of the luxA and luxB genes from mesophilic V. harveyi MAV (B 392), with minor exceptions. The sequence differences result in 5 amino acid substitutions in the α subunit polypeptide and 7 amino acid substitutions in the β subunit polypeptide. Escherichia coli cells grown on solid medium and expressing CTP5 or MAV luxAB genes emit similar amounts of light at 37° C, while at 42° C cells containing CTP5 luxAB genes show more than tenfold increased bioluminescence compared to cells with MAV luxAB genes. When grown in liquid medium E. coli cells with CTP5 or MAV luxAB genes emit equivalent amounts of light at 37° C; however, in liquid medium at 42° C cells containing CTP5 luxAB genes show only three times higher bioluminescence than cells with MAV luxAB genes. Expression of T7 promoter-linked hybrid luxAB transcriptional units luxA CTP5-luxB MAV and luxA MAV-luxB CTP5 in E. coli reveals that (i) the MAV luxB gene product is responsible for the decreased activity of MAV luciferase at 42° C; (ii) the CTP5 luxB gene encodes the information required for most of the increased activity of CTP5 luciferase relative to MAV luciferase at 42° C; and (iii) E. coli cells containing MAV luxB gene show an increase in bioluminescence when grown in liquid medium at 42° C, which coincides with elevated GroEL chaperonin levels. The MAV-CTP5 hybrid luciferases, with amino acid changes at known positions, provide a sensitive and simple system to study temperature-dependent assembly of multi-subunit enzymes in vivo.
Similar content being viewed by others
References
Baldwin TO, Berends T, Bunch TA, Holzman TF, Rausch SK, Shamansky L, Treat ML, Ziegler MM (1984) Cloning of the luciferase structural genes from Vibrio harveyi and expression of bioluminescence in Escherichia coli. Biochemistry 23:3663–3667
Baldwin TO, Devine JH, Lin JW, Legocki R, Szalay A, Peabody DS, Bear DG (1987) Applications of the cloned bacterial luciferase genes luxA and luxB to the study of transcriptional promoters and terminators. In: Scholmerich J, Andreseen R, Kapp A, Ernst M, Woods WG (eds) Bioluminescence and chemiluminescence: New perspectives. Wiley, Chichester, pp 373–376
Baldwin TO, Devine JH, Heckel RC, Lin JW, Shadil GS (1989) The complete nucleotide sequence of the lux regulon of Vibrio fischeri and the luxABN region of Photobacterium leiognathi and the mechanism of control of bacterial bioluminescence. J Biolumin Chemilumin 4:326–341
Belas R, Mileham A, Cohn D, Hilmen M, Simon M, Silverman M (1982) Bacterial bioluminescence: isolation and expression of the luciferase genes from Vibrio harveyi. Science 218:791–793
Boyer MW, Roulland-Dussoix D (1969) A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol 41:121–136
Buchner J, Schmidt M, Fuchs M, Jaenicke R, Rudolph R, Schmid FX, Kiefhaber T (1991) GroE facilitates refolding of citrate synthase by suppressing aggregation. Biochemistry 30:1586–1591
Cline TW, Hastings JW (1972) Mutationally altered luciferase. Implication for subunit functions. Biochemistry 11:3359–3370
Cohn DM, Mileham AJ, Simon MI, Nealson KH, Rausch SK, Bonam D, Baldwin TO (1985) Nucleotide sequence of the luxA gene of Vibrio harveyi and the complete amino acid sequence of the a subunit of bacterial luciferase. J Biol Chem 260:6139–6146
Colepicolo P, Cho KW, Poinar GO, Hastings JW (1989) Growth and luminescence of the bacterium Xenorhabdus luminescens from a human wound. Appl Environ Microbiol 55:2601–2606
Engebrecht J, Nealson K, Silverman M (1983) Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 32:773–781
Escher A, O'Kane DJ, Lee J, Szalay AA (1989) Bacterial luciferase αβ fusion protein is fully active as a monomer and highly sensitive in vivo to elevated temperature. Proc Natl Acad Sci USA 86:6528–6532
Escher A, O'Kane DJ, Szalay AA (1991) Engineering of a bacterial luciferase alpha-beta fusion protein with enhanced activity at 37° C in Escherichia coli. In: Stanley PE, Kricka LG (eds) Bioluminescence and chemiluminescence: Current status. Wiley, Chichester, pp 15–18
Fayet O, Louarn J-M, Georgopoulos C (1986) Suppression of the Escherichia coli dnaA46 mutation by amplification of the groES and groEL genes. Mol Gen Genet 202:435–445
Foran DR, Brown MW (1988) Nucleotide sequence of the luxA and luxB genes of the bioluminescent marine bacterium Vibrio fischeri. Nucleic Acids Res 16:177
Goloubinoff P, Gatenby AA, Lorimer GH (1989a) GroE heat shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature 337:44–47
Goloubinoff P, Christeller JT, Gatenby AA, Lorimer GH (1989b) Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfolded state depends on two chaperonin proteins and Mg-ATP. Nature 342:884–889
Illarionov BA, Protopopova MV, Farginov VA, Mertvetsov NP, Gitelson II (1988) Nucleotide sequence of genes of the luciferase alpha-subunits and beta-subunits from Photobacterium leiognathi. Bioorgan Khim 14:412–415
Johnston TC, Thompson RB, Baldwin TO (1986) Nucleotide sequence of the luxB gene of Vibrio harveyi and the complete amino acid sequence of the β subunit of bacterial luciferase. J Biol Chem 261:4805–4811
Johnston TC, Rucker EB, Cochrum L, Hruska KS, Vandegrift V (1990) The nucleotide sequence of the luxA and luxB genes of Xenorhabdus luminescens Hm and a comparison of the amino acid sequences of luciferases from four species of bioluminescent bacteria. Biochem Biophys Res Commun 170:407–415
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Matthews BW, Nicholson H, Becktel WJ (1987) Enhanced protein thermostability from site-directed mutations that decrease the entropy of unfolding. Proc Natl Acad Sci USA 84:6663–6667
Meighen EA, Bartlet I (1980) Complementation of subunits from different bacterial luciferases. J Biol Chem 255:11181–11187
Messing J, Crea R, Seeburg PH (1981) A system for shotgun DNA sequencing. Nucleic Acids Res 9:309–321
Norrander J, Kempe T, Messing J (1983) Construction of improved M13 vectors using oligodeoxynucleotide directed mutagenesis. Gene 26:101–106
Olsson O, Koncz C, Szalay AA (1988) The use of the luxA gene of the bacterial luciferase operon as a reporter gene. Mol Gen Genet 215:1–9
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schlesinger MJ (1990) Heat shock proteins. J Biol Chem 265:12111–12114
Sugihara J, Baldwin TO (1988) Effects of 3′ end deletions from the Vibrio harveyi luxB gene on luciferase subunit folding and enzyme assembly: generation of temperature-sensitive polypeptide folding mutants. Biochemistry 27:2872–2880
Szittner R, Meighen E (1990) Nucleotide sequence, expression, and properties of luciferase coded by lux genes from a terrestrial bacterium. J Biol Chem 265:16581–16587
Van Dyk TK, Gatenby AA, LaRossa RA (1988) Demonstration by genetic suppression of interaction of GroE products with many proteins. Nature 342:451–453
Xi L, Ki-Woong C, Tu S-C (1991) Cloning and nucleotide sequences of lux genes and characterization of luciferase of Xenorhabdus luminescens from a human wound. J Bacteriol 173:1399–1405
Author information
Authors and Affiliations
Additional information
Communicated by A. Kondorosi
Rights and permissions
About this article
Cite this article
Escher, A., O'Kane, D.J. & Szalay, A.A. The β subunit polypeptide of Vibrio harveyi luciferase determines light emission at 42° C. Molec. Gen. Genet. 230, 385–393 (1991). https://doi.org/10.1007/BF00280295
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00280295