Summary
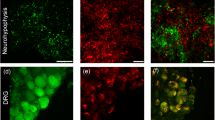
We examined opioid binding in fractions with disconnected nerve endings (secretosomes) which were prepared from porcine neurohypophyses by centrifugation in a discontinuous Percoll gradient. Specific (= displaceable) binding was observed with 3H-etorphine and with 3H-diprenorphine, two ligands with low selectivity for distinct opiate receptor sub-classes. No displaceable binding was found with the prototypic mu- and delta-ligands 3H-dihydromorphine and 3H-(D-Ala, D-Leu) enkephalin. Displacement of 3H-diprenorphine binding was almost absent with the selective mu- and delta-ligands morphiceptin and ICI-174864. Partial displacement occurred with the selective kappa-ligand U-50488 and with dynorphin (1–8). Binding of 3H-etorphine was stereo-specific. 3H-diprenorphine binding was saturable with a KD between 2 and 4 nM. Maximum of opiate binding activity was detected in the fractions with accumulated secretosomes. By autoradiography specific 3H-diprenorphine binding is shown to be mainly associated with secretosomes. In imunocytochemical preparations an oxytocin antibody was immunoreactive in 85% of the secretosomes in the fraction with highest opiate binding. These fractions in radioimmunoassays exhibited the largest contents in oxytocin and low vasopressin levels. The data therefore suggest that in the porcine neurohypophysis opioid binding sites of the kappa-type occur in secretory endings presumably of the oxytocin type.
Similar content being viewed by others
References
Baker RV, Hope DB (1976) The effect of gradual changes in temperature on the release of hormones from nerve endings isolated from bovine neural lobes. J Neurochem 27: 197–202
Bicknell RJ, Leng G (1982) Endogenous opiates regulate oxytocin but not vasopressin secretion from the neurohypophysis. Nature 298: 161–162
Bicknell RJ, Chapman C, Leng G (1985) Neurohypophyseal opioids and oxytocin secretion: source of inhibitory opioids. Exp Brain Res 60: 192–196
Blackett NM, Parry DM (1977) A simplified method of “hypothetical grain” analysis of electron microscope autoradiographs. J Histochem Cytochem 25: 206–214
Bunn SJ, Hanley MR, Wilkin GP (1985) Evidence for a kappaopioid receptor on pituitary astrocytes: an autoradiographic study. Neurosci Lett 55: 317–323
Castanas E, Bourhim N, Giraud P, Boudouresque F, Cantau P, Oliver C (1985) Interaction of opiates with opioid binding sites in the bovine adrenal medulla: II. Interaction with kappa sites. J Neurochem 45: 688–699
Castanas E, Blanc D, Bourhim N, Cupo A, Cantau P, Giraud P (1986) Reassessment of opioid binding sites in the rat brain. Neuropeptides 7: 369–380
Chang K-J, Killian A, Hazum E, Cuatrecasas P, Chang J-K (1981) Morphiceptin (NH4-Tyr-Pro-Phe-Pro-CONH2): a potent and specific agonist for morphine (mu) receptors. Science 212: 75–77
Clarke G, Wood P, Merrick L, Lincoln DW (1979) Opiate inhibition of peptide release from neurohumoral terminals of hypothalamic neurones. Nature 282: 746–748
Corbett A, Paterson SJ, McKnight AT, Magnan J, Kosterlitz HW (1982) Dynorphin 1–8 and dynorphin 1–9 are ligands of the kappa subtype of opiate receptor. Nature (Lond) 299: 79–81
Cotton R, Giles MG, Miller L, Shaw JS, Timms D (1984) ICI-174864: a highly selective antagonist for the opioid delta-receptor. Eur J Pharmacol 97: 331–332
Falke N, Martin R (1986) Characterization and localization of opioid binding sites in a rat neurohypophysial fraction enriched in secretory nerve endings. Neuroendocr Perspect 5: 291–295
Gaymann W, Martin R (1987) A re-examination of the localization of immunoreactive dynorphin (1–8), Leu-enkephalin and Met-enkephalin in the rat neurohypophysis. Neuroscience 20: 1069–1080
Gerstberger R, Barden N (1986) Dynorphin (1–8) binds to opiate kappa receptors in the neurohypophysis. Neuroendocrinology 42: 376–382
Herkenham M, Rice RC, Jacobson RE, Rothman RB (1986) Opiate receptors in rat pituitary are confined to the neural lobe and are exclusively kappa. Brain Res 382: 365–371
Iversen LL, Iversen SD, Bloom FD (1980) Opiate receptors influence vasopressin release from nerve terminals in rat neurohypophysis. Nature (London) 284: 350–351
Knepel W, Meyer DK (1983) The effect of naloxone on vasopressin release from rat neurohypophysis incubated in vitro. J Physiol 341: 507–515
Lightman SL, Iversen LL, Forsling ML (1982) Dopamine and (D-Ala2, D-Leu3) enkephalin inhibit the electrically stimulated neurohypophyseal release of vasopressin in vitro: evidence for calcium-dependent opiate action. J Neurosci 2: 78–81
Lightman SL, Ninkovic M, Hung SP, Iversen LL (1983) Evidence for opiate receptors on pituicytes. Nature (London) 305: 235–237
Lowry DH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
Martin R, Voigt KH (1981) Enkephalins co-exist with oxytocin and vasopressin in nerve terminals of rat neurohypophysis. Nature (London) 289: 502–504
Mayor HD, Hampton JC, Rosario B (1961) A simple method for removing the resin from epoxy embedded tissue. J Cell Biol 9: 909–910
Nagy A, Delgado-Escueta AV (1984) Rapid preparation of synaptosomes from mammalian brain using nontoxic isoosmotic gradient material (Percoll). J Neurochem 43.4: 1114–1123
Nordmann JJ, Dayanithi G, Cazalis M (1986) Do opioid peptides modulate, at the level of the nerve endings, the release of neurohypophysial hormones? Exp Brain Res 61: 560–566
Simantov R, Snyder SH (1977) Opiate receptor binding in the pituitary gland. Brain Res 124: 178–184
Vanderhaeghen JJ, Lotstra F, Liston DR, Rossier J (1983) Proenkephalin, (Met)enkephalin, and oxytocin immunoreactivities are colocalized in bovine hypothalamic magnocellular neurones. Proc Natl Acad Sci (USA) 80: 5139–5143
Von Voigtlander PF, Lahti RA, Ludens JH (1983) U-50,488: A selective and structurally novel non-mu (kappa) opioid agonist. J Pharmacol Exp Ther 224.1: 7–12
Wamsley JK, Zarbin MA, Young WS, Kuhar MJ (1982) Distribution of opiate receptors in the monkey brain: an autoradiographic study. Neuroscience 7.3: 595–613
Watson SJ, Akil H, Fischli W, Goldstein A, Zimmerman E, Nilaver G, Van Wimersma Greidanus TB (1982) Dynorphin and vasopressin: common localization in magnocellular neurons. Science 216: 85–87
Whitnall MH, Gainer H, Cox BM, Molineaux CJ (1983) Dynorphin-A1–8 is contained within vasopressin neurosecretory vesicles in rat pituitary. Science 222: 1137–1139
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Falke, N., Martin, R. Opiate binding differentially associated with oxytocin and vasopressin nerve endings from porcine neurohypophyses. Exp Brain Res 70, 145–154 (1988). https://doi.org/10.1007/BF00271856
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00271856