Summary
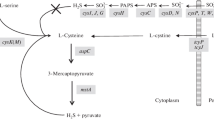
It was found that in Aspergillus nidulans the enzymes of the sulfate assimilation pathway and O-acetylhomoserine sulfhydrylase are under cysteine- and/or homocysteine, but not methionine- or S-adenosylmethionine-mediated regulation. These enzymes are repressed when the cells are grown in the presence of cysteine or homocysteine even in conditions where cysteine cannot be a precursor of homocysteine and vice versa. This was demonstrated by using mutants with impaired cystathionine cleavage enzymes. Thus, these two amino acids can substitute each other as the regulatory effectors. The addition of methionine causes repression only in conditions when it can be metabolized to homocysteine. The mutant cysA1 with a block at the serine transacetylase step is a prototroph owing to the existence of an alternative pathway for cysteine synthesis involving the enzymes: homocysteine synthase, cystathionine β-synthase and γ-cystathionase. All the three enzymes as well as those of the sulfate assimilation pathway are derepressed in this mutant. CysA1 mutation supresses the meth55 mutant blocked at β-cystathionase owing to the derepression of homocysteine synthase, so that the cystathionine cleavage step is bypassed. The results indicate that the pathway involving cystathionine formation is the main one for methionine biosynthesis in A. nidulans. The pathway involving homocysteine synthase is an alternative-conditional one, physiologically effective only when this enzyme is derepressed.
Similar content being viewed by others
References
Ahmed, A.: Mechanism of repression of methionine biosynthesis in Escherichia coli. Molec. gen. Genet. 123, 299–324 (1973)
Bessman, S. P., Kappanyi, Z. H., Wapnir, R. A.: A rapid method for homocysteine assay in physiological fluids. Analyt. Biochem. 18, 213–219 (1967)
Bradfield, G., Somerfield, P., Meyn, T., Holby, M., Babcock, D., Bradley, D., Segel, I. H.: Regulation of sulfate transport in filamentous fungi. Plant Physiol. 46, 720–727 (1970)
Cavallini, D., de Marco, C., Mondovi, B.: Chromatographic evidence on the occurence of thiotaurine in the urine of rats fed with cysteine. J. biol. Chem. 234, 854–857 (1959)
Cherest, H., Eichler, F., and de Robichon-Szulmajster, H.: Genetic and regulatory aspects of methionine biosynthesis in Saccharomyces cerevisiae. J. Bact. 97, 328–336 (1969)
Cherest, H., Surdin-Kerjan, Y., de Robichon-Szulmajster, H.: Methionine-mediated repression in Saccharomyces cerevisiae: a pleiotropic regulatory system involving methionyl transfer ribonucleic acid and product of gene eth2. J. Bact. 106, 758–772 (1971)
Cherest, H., Surdin-Kerjan, Y., Antoniewski, J., de Robichon-Szulmajster, H.: S-adenosyl methionine-mediated repression of methionine biosynthetic enzymes in Saccharomyces cerevisiae. J. Bact. 114, 928–933 (1973)
Cove, D. J.: The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. biophys. Acta (Amst.) 113, 51–56 (1966)
Delavier-Klutchko, C., Flavin, M.: Enzymatic synthesis and cleavage of cystathionine in fungi and bacteria. J. biol. Chem. 240, 2537–2549 (1965)
Duerre, J. A.: In vivo and in vitro metabolism of S-adenosyl-L-homocysteine by Saccharomyces cerevisiae. Arch. Biochem. Biophys. 124, 422–430 (1968)
Flavin, M., Slaughter, C.: The derepression and function of enzymes of reverse trans-sulfuration in Neurospora. Biochem. biophys. Acta (Amst.) 132, 406–411 (1967)
Gajewski, W., Litwińska, J.: Methionine loci and their suppressors in Aspergillus nidulans. Mol. gen. Genet. 102, 210–220 (1968)
Greene, R. C., Su, C. H., Holloway, C. T.: S-adenosylmethionine synthetase deficient mutants of Escherichia coli K12 with impaired control of methionine biosynthesis. Biochem. biophys. Res. Commun. 38, 1120–1126 (1970)
Holloway, C. T., Greene, R. C., Su, C. H.: Regulation of S-adenosylmethionine synthase in Escherichia coli. J. Bact. 104, 734–747 (1970)
Kerr, D. S.: O-acetylhomoserine sulfhydrylase from Neurospora: purification and consideration of its function in homocysteine and methionine synthesis. J. biol. Chem. 246, 95–102 (1971)
Kerr, D. S., Flavin, M.: Synthesis of cystathionine from O-acetylhomoserine in Neurospora: a step in methionine biosynthesis. Biochem. biophys. Res. Commun. 31, 124–130 (1968)
Kerr, D. S., Flavin, M.: The regulation of methionine synthesis and the nature of cystathionine γ-synthase in Neurospora. J. biol. Chem. 245, 1842–1855 (1970)
Kredich, N. M., Tomkins, G. N.: The enzyme synthesis of L-cysteine in E. coli and S. typhimurium. J. biol. Chem. 241, 4955–4965 (1966)
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J.: Protein measurement with Folin phenol reagent. J. biol. Chem. 193, 265–275 (1951)
Nagai, S., Flavin, M.: Acetylhomoserine, an intermediate in the fungal biosynthesis of methionine. J. biol. Chem. 242, 3884–3895 (1967)
Paszewski, A., Grabski, J.: Studies on β-cystathionase and O-acetylhomoserine sulfhydrylase as the enzymes of alternative methionine biosynthetic pathways in Aspergillus nidulans. Acta biochim. pol. 20, 159–167 (1973)
Pieniążek, N. J., Stępień, P. P., Paswewski, A.: An Aspergillus nidulans mutant lacking cystathionine β-synthase: identity of L-serine sulfhydrylase with cystathionine β-synthase and its distinctness from O-acetyl-L-erine sulfhydrylase. Biochim. biophys. Acta (Amst.) 297, 37–47 (1973a)
Pieniążek, N. J., Bal, J., Balbin, E., Stępień, P. P.: Two pathways for cysteine biosynthesis in Aspergillus nidulans. Molec. gen. Genet. (1974) in press
Pieniążek, N. J., Kowalska, I. M., Stępień, P. P.: Deficiency in methionine adenosyltransferase resulting in limited repressibility of methionine biosynthetic enzymes in Aspergillus nidulans. Molec. gen. Genet. 126, 367–374 (1973b)
Savin, M. A., Flavin, M.: Cystathionine synthesis in yeast: an alternative pathway for homocysteine biosynthesis. J. Bact. 112, 299–303 (1972)
Schlenk, F., Doinko, J. L., Svihla, G.: The accumulation and intracellular distribution of biological sulfonium compounds in yeast. Arch. Biochem. Biophys. 140, 228–236 (1970)
Siegel, L. M.: A direct microdetermination for sulfide. Analyt. Biochem. 11, 126–132 (1965)
Surdin-Kerjan, Y., Cherest, H., de Robichon-Szulmajster, H.: Relationship between methionyl transfer ribonucleic acid cellular content and synthesis of methionine enzymes in Saccharomyces cerevisiae. J. Bact. 113, 1156–1160 (1970)
Vito, P. C. de, Dreyfuss, J.: Metabolic regulation of adenosine triphosphate sulfurylase in yeast. J. Bact. 88, 1341–1348 (1964)
Wiebers, J. L., Garner, H. R.: Acyl derivatives of homoserine as substrates for homocysteine synthesis in Neurospora crassa, yeast and Escherichia coli. J. biol. Chem. 242, 5644–5649 (1967)
Author information
Authors and Affiliations
Additional information
Communicated by F. Kaudewitz
Rights and permissions
About this article
Cite this article
Paszewski, A., Grabski, J. Regulation of S-amino acids biosynthesis in Aspergillus nidulans. Molec. Gen. Genet. 132, 307–320 (1974). https://doi.org/10.1007/BF00268571
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00268571