Summary
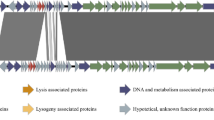
Genetically marked λ and P22 phages were recombined in Escherichia coli-Salmonella typhimurium hybrid WR4028, a host sensitive to infection by both of these phages. Hybrid phages that acquired the immC region of P22, but retained the genes for the λ protein coat were selected on WR4027 (λ), a λ-immune, P22-resistant derivative of WR4028. In these λimmP22 hybrids, at least the c through P genes of λ were replaced with functionally related P22 genes. Phage recombinants with more extensive regions of the P22 genome were selected on the double lysogen WR4027 (λ, λimmP22). One such hybrid, λimmP22dis, was determined by heteroduplex analysis to contain approximately 40% of the P22 genome. Genetic studies established that λimmP22dis possesses the two widely separated immunity control regions of P22 (immC and immI) and that these loci are expressed in E. coli K-12 lysogenic for λimmP22dis. In addition, λimmP22dis contains the P22 a1 locus responsible for somatic 0–1 antigen conversion in Salmonella. Although the λimmP22dis phage particle has the λ head and tail, the phage genome also carries P22 tail gene 9 as evidenced by the production of free P22 tails. It also has the P22 att site as indicated by the integration of the λimmP22dis prophage near the proA locus on the bacterial chromosome.
Similar content being viewed by others
References
Akiyoshi, H., Yamamoto, N.: Genetic evolution of bacteriophage II. Physical length of the homologous region in a hybrid between serologically and morphologically unrelated phagen. Proc. nat. Acad. Sci. (Wash.) 66, 385–389 (1970a)
Akiyoshi, H., Yamamoto, N.: DNA-DNA hybridization at low temperature using DNA chemically labelled with C14-dimethyl sulfate. Biochem. biophys. Res. Commun. 38, 915–920 (1970b)
Baron, L.S., Gemski, P., Jr., Yamamoto, N.: Sensitivity of Salmonella hybrids to phage recombinants between λ and P22. Abst. Ann. Meet., A.S.M. p. 243 (1975)
Baron, L.S., Ryman, I.R., Johnson, E.M., Gemski, P., Jr.: Lytic replication of coliphage lambda in Salmonella typhosa hybrids. J. Bact. 110, 1022–1031 (1972)
Bezdek, M., Amati, P.: Evidence for two immunity regulator systems in temperate bacteriophage P22 and L. Virology 36, 701–703 (1968)
Botstein, D., Chan, R.K., Waddell, C.L.: Genetics of bacteriophage P22 II. Gene order and gene function. Virology 49, 268–282 (1972)
Botstein, D., Herskowitz, I.: Properties of hybrids between Salmonella phage P22 and coliphage λ. Nature (Lond) 251, 584–589 (1974)
Botstein, D., Lew, K.K., Jarvik, V., Swanson, C.A., Jr.: Role of antirepressor in the bipartite control of repression and immunity by bacteriophage P22. J. molec. Biol. 91, 439–462 (1975)
Botstein, D., Matz, M.J.: A recombination function essential to the growth of bacteriophage P22. J. molec. Biol. 54, 417–440 (1972)
Campbell, A.: Genetic structure. In: The bacteriophage lambda (Hershey, A.D., ed.), pp. 13–14. New York: Cold Spring Harbor Laboratories 1971
Chan, R.K., Botstein, D.: Genetics of bacteriophage P22 I. Isolation of prophage deletions which affect immunity to superinfection. Virology 49, 257–267 (1972)
Cohen, D., A variant of phage P2 originating in Escherichia coli, strain B. Virology 7, 112–126 (1959)
Cowie, D.B., Szafranski, P.: Thermal chromatography of DNA-DNA reactions. Biophs. J. 7, 567–584 (1967)
Davies, R.W., Davidson, N.: Electron microscope visualization of deletion mutations. Proc. nat. Acad. Sci. (Wash.) 60, 243–254 (1968)
Davies, R.W., Simon, M., Davidson, N.: Electron microscope heteroduplex methods for mapping regions of base sequence homology in nucleic acids. In: Methods in enzymology (Grossman, L., Moldave, K., eds.), 21, pp. 413–428. New York: Academic Press 1971
Dove, W.F.: The genetics of the lamboid phages. Ann. Rev. Genet. 2, 305–340 (1968)
Falkow, S., Happala, D.K., Silver, R.P.: Relationships between extrachromosomal elements. In: Bacterial episomes and plasmids (Wolstenholme, G.E.W., O'Connor, M., eds.), pp. 36–162. London: J & A Churchill, LTD. 1969
Friedman, D.I., Baron, L.S.: Genetic characterization of bacterial locus involved in the activity of N gene expression. Virology 58, 141–148 (1974)
Friedman, D.I., Baumann, M., Baron, L.S.: Cooperative effects of bacterial mutations affecting N gene expresion. I. Isolation and characterization of a nusB mutant. Virology 73, 119–127 (1976)
Gemski, P., Jr., Baron, L.S., Yamamoto, N.: Formation of hybrids between coliphage λ and Salmonella phage P22 with a Salmonella typhimurium hybrid sensitive to these phages. Proc. nat. Acad. Sci. (Wash.) 69, 3110–3114 (1972)
Gough, M.: Second locus of bacteriophage P22 necessary for the maintenance of lysogeny. J. Virol. 2, 992–998 (1968)
Gough, M., Scott, J.V.: Location of the prophage conversion gene of P22. Virology 50, 603–605 (1972)
Hershey, A.D., Burgi, E., Ingraham, L.: Conesion of DNA molecules isolated from phage lambda. Proc. nat. Acad. Sci. (Wash.) 49, 748–755 (1963)
Israel, J.V., Anderson, T.F., Levine, M.: In vitro morphogenesis of phage P22 from heads and base-plate parts. Proc. nat. Acad. Sci. (Wash.) 57, 284–291 (1967)
Kaiser, D.: Lambda DNA replication. In: The bacteriophage lambda (Hershey, A.D., ed.), pp. 195–210. New York: Cold Spring Harbor Laboratories 1971
Levine, M., Curtiss, R.: Genetic fine structure of the C region and the linkage map of phage P22. Genetics 46, 1573–1580 (1961)
Levine M., Truesdell, S., Ramakrishnan, T., Bronson, M.J.: Dual control of lysogeny of bacteriophage P22: An antirepressor locus and its controlling elements. J. molec. Biol. 91, 421–438 (1975)
Oppenheim, A., Katzir, N., Oppenheim, A.B.: The product of gene P of coliphage λ. Virology 79, 437–441 (1977)
Rhoades, M., MacHattie, L.A., Thomas, C.A., Jr.: The P22 bacteriophage DNA molecule I. The mature form. J. molec. Biol. 37, 21–40 (1968)
Roth, J.R., Hoppe, I.: Specialized transduction by Salmonella phage P22. Genetics 71, 53 (1972)
Skalka, A., Hanson, P.: Comparisons of the distribution of nucleotides and common sequences in deoxyribonucleic acid from selected bacteriophages. J. Virol. 9, 583–593 (1972)
Smith, H.O.: Defective phage formation by lysogens of integration deficient phage P22 mutants. Virology 34, 203–223 (1968)
Smith, H.O., Levine, M.: Gene order in prophage P22. Virology 27, 229–231 (1965)
Smith, H.O., Stocker, B.A.D.: Mapping of prophage P22 in Salmonella typhimurium. Virology 28, 413–419 (1966)
Thomas, C.A., MacHattie, L.A.: The anatomy of viral DNA molecules. Ann. Rev. Biochem. 36, 485–518 (1967)
Westmoreland, B.C., Szybalski, W., Ris, H.: Mapping of deletions and substitutions in heteroduplex DNA molecules of bacteriophage lambda by electron microscopy. Science 163, 1343–1348 (1969)
Ushijima, N., Yamamoto, N., Gemski, P., Jr., Baron, L.S.: Genetic map of P22-λ hybrids. Abst. Ann. Meet. A.S.M. p. 243 (1975)
Yamagami, H., Yamamoto, N.: Contribution of the bacterial recombination function of replication of bacteriophage P22. J. molec. Biol. 53, 281–285 (1970)
Yamamoto, N.: The origin of bacteriophage P221. Virology 33, 545–547 (1967)
Yamamoto, N., Ushijima, N., Gemski, P., Baron, L.S.: Genetic studies of hybrids between coliphage lambda and Salmonella phage P22. Genetic analysis of P22-lambda hybrid class. Molec. gen. Genet. 155, 117–122 (1977)
Yamamoto, N., Weir, M.L.: Genetic relationships between serologically unrelated bacteriophages P22 and P22lb. Virology 28, 168–160 (1966a)
Yamamoto, N., Weir, M.L.: Boundary structure between homologous and nonhomologous regions in serologically unrelated bacteriophages P22 and P22l. I. Mapping of recombination found in cultures of double lysogenic strains for P22h and P221. Virology 28, 325–330 (1966b)
Young, B.G., Fukazawa, Y., Hartman, P.: A P22 bacteriophage mutant defective in antigen conversion. Virology 23, 279–283 (1964)
Young, B.G., Hartman, P.E.: Sites of P22 and P221 prophage integration in Salmonella typhimurium. Virology 28, 265–270 (1966)
Zinder, N.: Lysogenization and superinfection immunity in Salmonella. Virology 5, 291–326 (1958)
Author information
Authors and Affiliations
Additional information
Communicated by T. Yura
Rights and permissions
About this article
Cite this article
Yamamoto, N., Wohlhieter, J.A., Gemski, P. et al. λimmP22dis: A hybrid of coliphage λ with both immunity regions of Salmonella phage P22. Molec. Gen. Genet. 166, 233–243 (1978). https://doi.org/10.1007/BF00267614
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00267614