Abstract
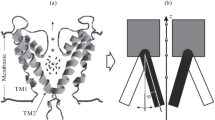
Electric dipoles placed side by side attract each other if antiparallel and repel each other if parallel. The hydrophobic α-helical sections of proteins that span membranes are known to possess large electric dipole moments. The first part of the paper consists of a calculation of the interaction energies between such helices including screening effects. Interaction energies remain comparable with a typical thermal energy of KT up to separations of order 20 Å. In addition it is shown that, due solely to its dipole moment, an α-helix which completely spans the membrane has an energy up to 5 KT lower than one which terminates within the membrane width. The second part of the paper describes the electrical interaction of the charge structure of a membrane channel and the protein helices that surround the pore. The gating charge transfer that is measured when a voltage sensitive ion channel switches, means that the dipole moment of the ion channel changes. This in turn results in a change in the radial forces that act between the pore and the α-helices that surround it. A change in these radial forces which tend to open or to close the pore constitutes an electrically silent gating mechanism that must necessarily act subsequent to the gating charge transfer. The gating mechanism could consist of the radial translation of the neighbouring proteins or in their axial rotation under the influence of the torque that would act on a pair of approximately equidistant but oppositely directed α-helices. An attempt to calculate the interaction energy of a typical pore and a single α-helix spanning the membrane results in an energy of many times KT.
Similar content being viewed by others
References
Boheim G, Hanke W, Jung G (1983) Alamethicin pore formation: Voltage-dependent flip-flop of α-helix dipoles. Biophys Struct Mech 9:181–191
Changeux J, Devillers-Thiery A, Chemouilli P (1984) Acetylcholine receptor: an allosteric protein. Science 225:1335–1345
Conti F, Inoue I, Kukita F, Stuhmer W (1984) Pressure dependence of sodium gating currents in the squid giant axon. Eur Biophys J 11:137–147
Edmonds DT (1984) The ordered water model of membrane channels. In: Biological membranes, vol 5. Academic Press, New York, Chap 10
Hol WGJ, Duijnen PT van, Berendsen HJC (1978) The α-helix and the properties of proteins. Nature 273:443–446
Hol WGJ, Halie LM, Sander C (1981) Diples of the α-helix and β-sheet: their role in protein folding. Nature 294: 532–553
Keynes RD (1983) Voltage gated ion channels in the nerve membrane. Proc R Soc London Ser B220:1–30
Noda M, Shimizu S, Tanabe T, Takai T, Kayano T, Ikeda T, Takahashi H, Nakayama H, Kanaoka Y, Minamino N, Kangawa K, Matsuo H, Raftery MA, Hirose T, Inayama S, Hayashida H, Miyata T, Numa S (1984) Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature 312:121–127
Rogers NK, Sternberg MJE (1984) Electrostatic interactions in globular proteins: different dielectric models applied to the packing of α-helices. J Mol Biol 174:527–542
Schauf CL, Bullock JO (1979) Modifications of sodium channel gating in Myxicola giant axons by deuterium oxide, temperature and internal cations. Biophys J 27:193–208
Smythe WR (1939) In: Static and dynamic electricity. McGraw-Hill, New York, pp 193–195
Wada A (1976) The α-helix as an electric macro-dipole. Adv Biophys 9:1–63