Abstract
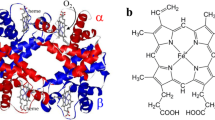
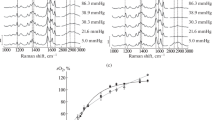
The dispersion of the depolarization ratio of two prominent Raman lines (1,375 cm−1 and 1,638 cm−1) of oxyhemoglobin-N-ethyl succinimide have been examined for pH values between pH=6.0 and 8.5. Both exhibit a significant pH dependence. Calculation of the Raman tensor in terms of a fifth-order time dependent theory provides information about the pH-dependence of parameters reflecting symmetry classified distortions of the prosthetic heme group. To correlate these distortions with the functional properties of the molecule the following protocol was used: 1) An allosteric model suggested by Herzfeld and Stanley (1974) has been applied to O2-binding curves measured at different pH values between 6.5 and 9.0. From this calculation one obtains both, the energy differences between different molecular conformations and the equilibrium constants of oxygen and proton binding. 2) A titration model was formulated relating each conformation of a molecule to a distinct set of distortion parameters of the heme group. 3) The distortion parameters resulting from the analysis of our Raman data were assigned as an effective value due to incoherent superposition of the distortion parameters related to the different titration states. The application of this procedure yields an excellent reproduction of the pH-dependent effective distortion parameters of both Raman lines investigated. It is shown that the protonation of two tertiary effector groups located in the β-subunits affect the symmetry of the heme in a contrary manner: the protonation of a His-residue (pK=8.2, probably His(FG4)β) causes a symmetric position of the proximal imidazole thus lowering the perturbations of the heme core. Further it influences the interaction between amino acid residues of the heme cavity and pyrrole side chains (probably Val (FG5)β-vinyl (pyrrole 3) thus causing a decrease of the distortions related to the peripheral part of the heme. In contrast, the protonation of Lys (EF6) β causes a tilt position of the proximal imidazole and an increase of asymmetric perturbations of the heme core, whereas the interaction between the pyrrole side chains and the heme cavity is weakened. Our results are consistent with stereochemical predictions of Moffat (1971) concerning the existence of an H-bond between His(FG4)β and Cys(F9)β.
Similar content being viewed by others
Abbreviations
- DPR:
-
depolarization ratio
- EP:
-
excitation profile
- HbA:
-
human hemoglobin
- oxyHb:
-
oxyhemoglobin
- NEM:
-
N-ethyl-maleimide
- NES:
-
N-ethyl-succimide
- BME:
-
Bis (N-maleimidodimethyl)ether
References
Arata Y, Seno Y, Jinay O (1988) A study on the quaternary structure change of hemoglobin in the ligation process. Biochim Biophys Acta 956:243–255
Abe M, Kitagawa T, Kyogoku Y (1978) Resonance Raman spectra in octaethylporphyrin-Ni(II) and mesodeuterated and 15N-substituted derivatives. II. A normal coordinate analysis. J Chem Phys 69:4526–4534
Brunner H, Mayer A, Sussner H (1972) Resonance Raman scattering on the Haem Group of Oxy- and Deoxyhaemoglobin. J Mol Biol 70:153–156
Brunzel U, Dreybrodt W, Schweitzer-Stenner R (1986) pH-dependent absorption in the B-and Q bands of oxyhemoglobin and chemically modified oxyhemoglobin (BME) at low Cl−-concentration. Biophys J 49:1069–1076
De Young A, Pennely R, Tan-Wilson A, Noble RW (1976) Kinetic studies on the binding affinity of human hemoglobin for the 4th carbon monoxide molecule L4. J Biol Chem 251:6692–6698
Friedman JM, Scott TW, Stepanowski RA, Ikedo-Saito M, Cone RL (1983) The iron-proximal histidine linkage and protein control of oxygen binding in hemoglobin. J Biol Chem 258:10564–10572
Gellin BT, Karplus M (1977) Mechanism of tertiary structural change in hemoglobin. Proc Natl Acad Sci USA 74:801–805
Herzfeld J, Stanley E (1974) A general approach to cooperativity and its application to the oxygen equilibrium of human hemoglobin and its effectors. J Mol Biol 82:231–265
Hsu MC (1970) Optical activity of heme proteins. PhD-thesis, Urbana, Illinois
Kilmartin JV, Fogg JH, Perutz MF (1980) Role of C-terminal histidine in the alkaline Bohr effect of human hemoglobin. Biochemistry 19:3189–3193
Koshland DE, Nemethy G, Filer D (1966) Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry 5:365–385
Kwiatkowski L, Noble RW (1982) The contribution of histidine (HC3) (146)β to the R-state Bohr effect in human hemoglobin. J Biol Chem 257:8891–8895
Loudon R (1973) The quantum theory of light. Clarendon Press, Oxford
Moffat JK (1971) Structure and functional properties of chemically modified horse hemoglobin. J Mol Biol 58:79–88
Monod J, Wyman J, Changeux JPC (1965) On the nature of allosteric transitions: a plausible model. J Mol Biol 12: 88–118
el Naggar S, Dreybrodt W, Schweitzer-Stenner R (1985) Haem-apoprotein interactions detected by resonance Raman scattering in Mb- and Hb-derivatives lacking the saltbridge His 146 β-Asp94β. Eur Biophys J 12:43–49
Perutz MF (1969) The haemoglobin molecule. Proc R Soc B 173:113–140
Perutz MF (1970) Stereochemistry of cooperative effects in haemoglobin. Nature (London) 228:726–734
Perutz MF, Kilmartin JV, Nishikura K, Fogg JH, Butler PJG (1980) Identification of residues contributing to the Bohr effect of human hemoglobin. J Mol Biol 138:649–670
Perutz MF, Gronenborn AM, Clore GM, Fogg JH, Shih Tb (1985) The pKa-values of two histidine residues in human hemoglobin, the Bohr effect and the dipole moment of the α-helix. J Mol Biol 183:491–498
Peticolas W, Nafie I, Stein P, Fanconi B (1970) Quantum theory of the intensities of molecular vibrational spectra. J Chem Phys 52:1576–1588
Schweitzer R (1983) Untersuchung von pH-induzierten Symmetrieverzerrungen der prosthetischen Gruppe im Hämoglobin durch resonante Ramanstreuung. Doctoral thesis, Bremen
Schweitzer-Stenner R, Dreybrodt W (1985) Excitation profiles and depolarization ratios of some prominent Raman lines in oxyhaemoglobin and ferrocytochrome c in the preresonant and resonant region of the Q-band. J Raman Spectrosc 16:111–123
Schweitzer-Stenner R, Dreybrodt W (1989) An extended MWC-model expressed in terms of the Herzyfeld-Stanley formalism applied to oxygen and carbonmonoxide binding curves of hemoglobin trout IV. Biophys J 55:691–701
Schweitzer-Stenner R, Dreybrodt W, el Naggar S (1984a) Investigation of pH-induced symmetry distortions of the prosthetic group in deoxyhemoglobin by resonance Raman scattering. Biophys Struct Mech 10:241–256
Schweitzer-Stenner R, Dreybrodt W, Wedekind D, el Naggar S (1984b) Investigation of pH-induced symmetry distortions of the prosthetic group in oxyhemoglobin by resonance Raman scattering. Eur Biophys J 11:61–76
Schweitzer-Stenner R, Wedekind D, Dreybrodt W (1986) Correspondence of the pK-values of oxyHb-titration states detected by resonance Raman scattering to kinetic data of ligand dissociation and association. Biophys J 49:1077–1088
Schweitzer-Stenner R, Wedekind D, Dreybrodt W (1989) Allosteric transitions in oxyHb trout IV detected by resonance Raman spectroscopy. Biophys J 55:703–705
Shaanan B (1983) Structure of human oxyhemoglobin at 2:1Å resolution. J Mol Biol 171:31–59
Shih Tb, Jones RT, Bonaventura J, Bonaventura C, Schneider RG (1984) Involvement of His HC3(146)β in the Bohr effect of human hemoglobin. Studies of native and N-ethylmaleimido-treated hemoglobin A and hemoglobin Cowntown (β146 His→Leu). J Biol Chem 259:967–974
Simon SR, Arndt DJ, Konigsberg WH (1971) Structure and functional properties of chemically modified horse hemoglobin. J Mol Biol 58:69–77
Warshel A (1977) Energy-structure correlation in metalloporphyrins and the control of oxygen binding by hemoglobin. Proc Natl Acad Sci USA 74:1789–1793
Wedekind D, Schweitzer-Stenner R, Dreybrodt W (1985) Heme-apoprotein interaction in the modified oxyhemoglobin-bis-(N-maleimidomethyl) ether and in oxyhemoglobin at high Cl−-concentration detected by resonance Raman scattering. Biochim Biophys Acta 830:224–232
Wedekind D, Brunzel U, Schweitzer-Stenner R, Dreybrodt W (1986) Correlation of pH-dependent resonance Raman and optical absorption data reflecting heme-apoprotein interaction in oxyhaemoglobin. J Mol Struct 143:457–460
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schweitzer-Stenner, R., Wedekind, D. & Dreybrodt, W. The influence of structural variations in the F- and FG-helix of the β-subunit modified oxyHb-NES on the heme structure detected by resonance Raman spectroscopy. Eur Biophys J 17, 87–100 (1989). https://doi.org/10.1007/BF00257106
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00257106