Summary
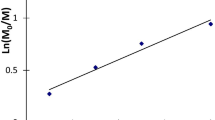
The use of a Ce (IV)-thiomalic acid (Ce4+-TMA) redox system as an initiator in the polymerization of acrylamide (M) in an aqueous medium has been investigated. The Ce4+ forms a 1∶1 complex with TMA, decomposing through free radical mechanism in an acid medium. From 5 to 15% conversion the rate equation is \(R_p = - \frac{{d[M]}}{{dt}} = k[M]^{3/2} [Ce^{4 + } ]^x [TMA]\) where Rp is the rate of polymerization and x is 0.5 or 1.0 depending upon the concentration of monomer. The overall energy of activation has been calculated to be 9.12 k cal. deg−1 mol−1(38.12 kJ/mol) in the investigated range of temperature (25°–40°C) the degree of polymerization (P) of the polymer is directly proportional to [M]. The number of average molecular weight of the polymer remains unaffected at lower concentration of Ce4+ and is found to decrease at higher concentrations.
In this investigation the kinetics and mechanism of the Ce(IV)-thiomalic acid (TMA) redox system to initiate the polymerization of acrylamide has been studied.
Acrylamide (E. Merck) was purified by usual methods. Thiomalic acid and eerie ammonium sulfate were used without purification. All solutions were p repared in twice distilled water.
The polymerization procedure adopted was similar to the one used by MISRA et al. [3, 4]. The polymerization was followed by quantitative estimation of the double bonds in acrylamide as described [8]. A small variable induction period was observed perhaps due tothe residual oxygen in the thiol solution. Curves were plotted after eliminating the induction period.
The average molecular weights of the p olymers were determined by viscosity measurements at 30°C in an aqueous medium using the relationship of DAINTON et al. [2]. \([\eta ] 30^\circ = 6.8 x 10^{ - 4} \bar Mn^{0.66} \)
Where [η]=intrinsic viscosity of the polymer solution and ¯Mn=molecular weight of the sample.
Similar content being viewed by others
References
ALEXANDER, W.A., MASH, C.J., and MCAULEY,A.: Talanta, 16, 535 (1969)
COLLINSON, E., DAINTON, F.S., and MCNAUGHTON, G.S.: Trans. Faraday Soc. 53, 489 (1957)
MISRA, G.S., SHUKLA, J.S., and NARAIN, H.: Makromol. Chem. 119, 74 (1968)
MISRA, G.S. and NARAIN, H.: Makromol. Chem. 113, 85 (1968)
MUKHTAR HUSSAIN, M. MISRA, SADANAND, and GUPTA, ARCHANA.: Makromol. Chem.177, 2919 (1976)
RIGGS, J.P. and RODRIGUEZ, F.: J.Polym. Sci. A-1(5) 3151 (1967)
SUBRAMANIAN, S.V., and SANTAPPA, M.: J.Polym.Sci. A–1(6), 493 (1968)
WALLACE, R.A. and YOUNG, D.G.: J. Polym.Sci. 4, 1176 (1966)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Misra, G.S., Dubey, G.P. Aqueous polymerization of acrylamide initiated by Ce(IV)-thiomalic acid redox system. Polymer Bulletin 1, 671–678 (1979). https://doi.org/10.1007/BF00255441
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00255441