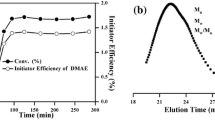
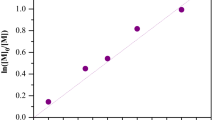
Abstract
Since cerium(IV) ammonium nitrate (CAN) could oxidize amidyl N–H moieties into nitrogen-centered radicals under proper conditions to initiate radical polymerization, CAN-acrylamide (AAm) redox-initiated radical homo/co-polymerization in a conventional or controlled manner was exploited with AAm as an intrinsically reducing inimer. CAN oxidized AAm to initiate conventional radical homopolymerization at 50–60 °C, and viscosity-average molecular weight (MW) of polyacrylamide (PAAm) steadily increased with conversion because of the oxidation of amidyl N–H moieties of in-chain -AAm- units, but MW was in a range from 104 to 105. CAN-AAm redox-initiated radical co-polymerization of N,N-dimethylacrylamide (DMAAm) proceeded readily under optimized conditions and formed PAAm-co-PDMAAm with MW in a range from 105 to 106, and increasing simultaneously with conversion. CAN-AAm redox-initiated controlled radical homopolymerization was performed with FeCl3 complexes as the deactivator, and the polymerization proceeded in a mechanism of reverse atom transfer radical polymerization (ATRP) and MW of PAAm increased in proportion to conversion. The PAAm chains contained a C–Cl terminal, which was confirmed by block co-polymerization with DMAAm via standard ATRP with PAAm as the macro-initiator.











Similar content being viewed by others
References
Kulicke W-M, Kniewske R, Klein J (1982) Preparation, characterization, solution properties and rheological behaviour of polyacrylamide. Prog Polym Sci 8(4):373–468. https://doi.org/10.1016/0079-6700(82)90004-1
Liu Yh, Jb Li, Ly Y, Zq S (2003) Study on kinetics of acrylamide polymerization initiated by potassium ditelluratocuprate (III) in alkaline medium. J Macromol Sci Part A Pure Appl Chem 40(10):1107–1117. https://doi.org/10.1081/MA-120024465
Zhang X, Liu W, Chen Y, Gong A (1999) Self-condensing vinyl polymerization of acrylamide. Polym Bull 43:29–34. https://doi.org/10.1007/s002890050529
Haworth S, Holker JR (1966) Cerium–initiated polymerisation of some vinyl compounds in polyamide fibres. J Soc Dyers Colour 82(7):257–264. https://doi.org/10.1111/j.1478-4408.1966.tb02721.x
Pradhan AK, Pati NC, Nayak PL (1982) Grafting vinyl monomers onto nylon 6. V. Graft copolymerization of methyl methacrylate onto nylon 6 with the tetravalent cerium-thiourea redox system. J Polym Sci Part A: Polym Chem 20(1):257–261. https://doi.org/10.1002/pol.1982.170200130
Samal RK, Nayak MC, Panda G, Suryanarayana GV (1982) Polymerization of acrylonitrile. Kinetics of the reaction initiated by the Ce(IV)/thioacetamide redox system. J Polym Sci Part A: Polym Chem 20(1):53–62. https://doi.org/10.1002/pol.1982.170200106
Takahashi T, Hori Y, Sato I (1968) Coordinated radical polymerization and redox polymerization of acrylamide by ceric ammonium nitrate. J Polym Sci, Part A: Polym Chem 6(8):2091–2102. https://doi.org/10.1002/pol.1968.150060807
Curvale RA, Cesco JC (2019) Intrinsic viscosity determination by “single-point” and “double-point” equations. Appl Rheol 19(5):53347–1. https://doi.org/10.3933/ApplRheol-19-53347
Fantin M, Isse AA, Gennaro A, Matyjaszewski K (2015) Understanding the Fundamentals of Aqueous ATRP and Defining Conditions for Better Control. Macromolecules 48(19):6862–6875. https://doi.org/10.1021/acs.macromol.5b01454
Fantin M, Isse AA, Venzo A, Gennaro A, Matyjaszewski K (2016) Atom Transfer Radical Polymerization of Methacrylic Acid: A Won Challenge. J Am Chem Soc 138(23):7216–7219. https://doi.org/10.1021/jacs.6b01935
Lorandi F, Fantin M, Wang Y, Isse AA, Gennaro A, Matyjaszewski K (2020) Atom Transfer Radical Polymerization of Acrylic and Methacrylic Acids: preparation of Acidic Polymers with various architectures. ACS Macro Lett 9(5):693–699. https://doi.org/10.1021/acsmacrolett.0c00246
Fu L, Simakova A, Fantin M, WangOrcid Y, Matyjaszewski K (2018) Direct ATRP of Methacrylic Acid with Iron-Porphyrin based catalysts. ACS Macro Lett 7(1):26–30. https://doi.org/10.1021/acsmacrolett.7b00909
Xue Z, He D, Xie X (2015) Iron-catalyzed atom transfer radical polymerization. Polym Chem 6(10):1660–1687. https://doi.org/10.1039/C4PY01457J
Poli R, Allan LEN, Shaver MP (2014) Iron-mediated reversible deactivation controlled radical polymerization. Prog Polym Sci 39(10):1827–1845. https://doi.org/10.1016/j.progpolymsci.2014.06.003
Li L, Cao YF, Gu CJ, Liang LX (2009) Reverse atom transfer radical polymerization of acrylamide catalyzed by iron(III) system. J Dalian Polytech Univ 28(4):281–284. https://doi.org/10.3969/j.issn.1674-1404.2009.04.013
Giz A, Çatalgil-Giz H, Alb A, Brousseau J-L, Reed WF (2001) Kinetics and mechanisms of acrylamide polymerization from absolute, online monitoring of polymerization reaction. Macromolecules 34(5):1180–1191. https://doi.org/10.1021/ma000815s
Kurenkov VF, Myagchenkov VA (1980) Effects of Reaction Medium on the Radical Polymerization and Copolymerization of Acrylamide. Eur Polymer J 16(12):1229–1239. https://doi.org/10.1016/0014-3057(80)90030-0
Lacík I, Chovancová A, Uhelská L, Preusser C, Hutchinson RA, Buback M (2016) PLP-SEC Studies into the Propagation Rate Coefficient of Acrylamide Radical Polymerization in Aqueous Solution. Macromolecules 49(9):3244–3253. https://doi.org/10.1021/acs.macromol.6b00526
Pascal P, Napper DH, Gilbert RG, Piton MC, Winnik MA (1990) Pulsed laser study of the propagation kinetics of acrylamide and methacrylamide in water. Macromolecules 23(24):5161–5163. https://doi.org/10.1021/ma00226a024
Seabrook SA, Tonge MP, Gilbert RG (2005) Pulsed laser polymerization study of the propagation kinetics of acrylamide in water. J Polym Sci, Part A: Polym Chem 43(7):1357–1368. https://doi.org/10.1002/pola.20605
Acknowledgement
This work was supported by the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Y., Zhai, G. Conventional and controlled radical polymerization redox-initiated by Cerium(IV) and Acrylamide as an intrinsically reducing inimer: a facile strategy to branched polyacrylamide. Polym. Bull. 79, 1751–1765 (2022). https://doi.org/10.1007/s00289-021-03587-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03587-z