Summary
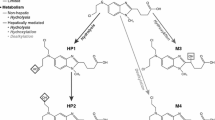
Ifosfamide (IF) pharmacokinetics and the plasma (NBP)-alkylating activity were determined in 33 patients with different tumours after the administration of IF as single-agent chemotherapy. All subjects had been phenotyped for debrisoquine oxidation. There is a lack of correlation between the debrisoquine metabolic ratio (DMR) and either the total plasma clearance of IF (CLIF) or the AUC of the plasma NBP-alkylating activity.
Similar content being viewed by others
References
Allen LM, Creaven PJ (1975) Pharmacokinetics of ifosfamide. Clin Pharmacol Ther 17:492
Brade W, Seeber S, Herdrich K (1986) Comparative activity of ifosfamide and cyclophosphamide. Cancer Chemother Pharmacol 18 (Suppl 2): S1-S9
Breimer DD (1983) Interindividual variations in drug disposition. Clinical implications and methods of investigation. Clin Pharmacokinet 8:371
Brock N (1983) Oxazaphosphorine cytostatics: structure activity relationships, selectivity and metabolism, regional detoxification. In: Structure-activity Relationships of Anti-tumour Agents, edited by D.N. Reinhoudt, T.A. Connors, H.M. Pinedo and K.W. van de Poll (Boston: Martinus Nijhoff Publishers), 239
Clark DWJ (1985) Genetically determined variability in acetylation and oxidation. Therapeutic implications. Drugs 29:342
Colvin M (1982) The comparative pharmacology of cyclophosphamide and ifosfamide. Semin Oncol 9:2
Connors TA, Cox PJ, Farmer PR, Foster AB, Jarman M (1974) Some studies of the active intermediates formed in the microsomal metabolites of cyclophosphamide and ifosfamide. Biochem Pharmacol 23:115
Eichelbaum M (1982) Defective oxidation of drugs: pharmacokinetic and therapeutic implications. Clin Pharmacokinet 7:1
Evans DAP, Mahgoub A, Sloan TP, Idle JR, Smith RL (1980) A family and population study of the genetic polymorphism of debrisoquine oxidation in a white British population. J Med Genet 17:102
Friedman OM, Boger E (1961) Colorimetric estimation of nitrogen mustards in aqueous media. Hydrolytic behaviour of bis (beta-chloroethyl) amine, nor HN2. Anal Chem 33:906
Hill DL, Laster WF Jr, Kirk MC, El Dareer S, Struck RF (1973) Metabolism of ifosfamide and production of a toxic ifosfamide metabolite. Cancer Res 33:1016
Juma FJ, Rogers HJ, Trounce JR (1979) Pharmacokinetics of cyclophosphamide and alkylating activity in urine after intravenous and oral administration. Br J Clin Pharmacol 8:209
Kalow W (1987) Genetic variation in the human hepatic cytochrome P-450 system. Eur J Clin Pharmacol 31:633
Lennard MS, Silas JH, Tucker GT (1977) Determination of debrisoquine and its 4-hydroxy metabolite in biological fluids by gas chromatography with flame-ionization and nitrogenselective detection. J Chromatogr 133:161
Lu AYH, West SB (1980) Multiplicity of mammalian microsomal cytochrome P-450. Pharmacol Rev 31:277
Mahgoub A, Idle JR, Dring LG, Lancaster R, Smith RL (1977) Polymorphic hydroxylation of debrisoquine in man. Lancet ii:584
Meyer UA, Gut J, Kronbach T, Skoda R, Meier UT, Dayer P (1986) The molecular mechanisms of common polymorphisms of drug oxidation—evidence for functional changes in cytochrome P-450 isoenzymes catalysing bufuralol and mephenytoin oxidation. Xenobiotica 16:449
Norpoth K (1976) Studies on the metabolism of ifosphosphamide (NSC-109724) in man. Cancer Treat Rep 60:437
Park BK (1982) Assessment of the drug metabolism capacity of the liver. Br J Clin Pharmacol 14:631
Siegel S (1956) Non parametric statistics for the behavioural sciences. McGraw-Hill Inc., New York
Sloan TP, Lancaster R, Shah RR, Idle JR, Smith RL (1983) Genetically determined oxidation capacity and the disposition of debrisoquine. Br J Clin Pharmacol 15:443
Talha MRZ, Rogers HJ (1984) Rapid gas chromatographic determination of ifosfamide in biological fluids. J Chromatogr 311:194
Van Dyk JJ, Falkson HC, Van der Merwe AM, Falkson G (1972) Unexpected toxicity in patients with ifosphamide. Cancer Res 32:921
Vesell ES (1977) Genetic and environmental factors affecting drug disposition in man. Clin Pharmacol Ther 22:659
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Philip, P.A., Lewis, L.D., James, C.A. et al. Ifosfamide plasma clearance in relation to polymorphic debrisoquine oxidation. Cancer Chemother. Pharmacol. 22, 321–324 (1988). https://doi.org/10.1007/BF00254239
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00254239