Summary
Purpose
Pyrotinib (PTN), an irreversible EGFR/HER2 dual tyrosine kinase inhibitor used for treating HER2-positive breast cancer, is primarily metabolized by cytochrome P450 (CYP)3A4 isozyme. Rifampicin (RIF) is a strong index CYP3A4 inducer. Therefore, the study aimed to elucidate the effect of RIF on PTN pharmacokinetics (PK) in Chinese healthy volunteers.
Methods
This phase I, open-label study investigated the effects of steady-state RIF administration on single-dose PK of PTN. 18 healthy participants were enrolled in this trial, who received a single oral dose of 400 mg of PTN on days 1 and 13, and were administrated with RIF 600 mg qd on days 6 through 16. RIF was administrated on an empty stomach, PTN were administrated orally in the morning 30 min after the start of the standard meal. Serial PK samples for PTN were collected on days 1 and days 13. Safety assessments were performed via clinical laboratory tests throughout the study.
Results
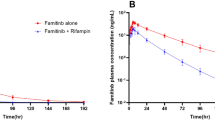
18 subjects were enrolled and 16 completed the study. RIF significantly reduced PTN exposure: Geometric least-squares mean ratios (90% CI) for PTN + RIF versus PTN alone were 0.04 (0.034,0.049), 0.04 (0.037,0.054), and 0.11 (0.09,0.124) for area under the curve from time zero to time of last quantifiable concentration (AUC0 − t), area under the curve from time zero to infinity (AUC0−∞ ), and maximum observed plasma concentration(Cmax), respectively. PTN alone and co-administered with RIF was well tolerated.
Conclusion
The exposure of PTN was significantly affected by the action of RIF. The findings suggest that concomitant strong CYP3A4 inducers should be avoided during PTN treatment. Concurrent administration of PTN and RIF was well tolerated.


Similar content being viewed by others
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Huang T, Luo X, Wu B et al (2020) Pyrotinib enhances the radiosensitivity of HER2overexpressing gastric and breast cancer cells[J]. Oncol Rep 44(6):2634–2644
Blair HA, Pyrotinib (2018) First Global Approval[J]. Drugs 78(16):1751–1755
Su B, Huang T, Jin Y et al (2021) Apatinib exhibits synergistic effect with pyrotinib and reverses acquired pyrotinib resistance in HER2-positive gastric cancer via stem cell factor/c-kit signaling and its downstream pathways[J]. Gastric Cancer 24(2):352–367
Fei Q (2019) Pyrotinib or Lapatinib Combined With Capecitabine in HER2-Positive Metastatic Breast Cancer With Prior Taxanes, Anthracyclines, and/or Trastuzumab: A Randomized, Phase II Study.[J]. J Clin oncology: official J Am Soc Clin Oncol 37(29):2610–2619
Xuhong JC, Qi XW, Zhang Y et al (2019) Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer[J]. Am J Cancer Res 9(10):2103–2119
Meng J, Liu XY, Ma S et al (2019) Metabolism and disposition of pyrotinib in healthy male volunteers: covalent binding with human plasma protein[J]. Acta Pharmacol Sin 40(7):980–988
Lin Y, Lin M, Zhang J et al (2020) Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis[J]. Cancer Res Treat 52(4):1059–1066
Li J, Rockich K, Yuska B et al (2020) An Open-Label Study to Assess the Effect of Itraconazole and Rifampin on Parsaclisib Pharmacokinetics When Administered Orally in Healthy Participants[J]. J Clin Pharmacol 60(11):1519–1526
Elin M, Svensson S, Murray MO, Karlsson et al (2015) Rifampicin and rifapentine significantly reduce concentrations of bedaquiline, a new anti-TB drug[J]. J Antimicrob Chemother 70:1106–1114
Niladri Chattopadhyay T, Kanacher M, Casjens et al (2018) CYP3A4-mediated effects of rifampicin on the pharmacokinetics of vilaprisan and its UGT1A1-mediated effects on bilirubin glucuronidation in humans.[J]. Br J Clin Pharmacol 84:2857–2866
Zhou X, Pant S, Nemunaitis J et al (2017) Effects of rifampin, itraconazole and esomeprazole on the pharmacokinetics of alisertib, an investigational aurora a kinase inhibitor in patients with advanced malignancies[J]. Investigational New Drugs
Yue L, Qian Z, Chao L et al Multiple administrations of itraconazole increase plasma exposure to pyrotinib in Chinese healthy adults[J]. Drug Design, Development and Therapy,2021:15 2485–2493
Acknowledgements
We would like to thank all the subjects who participated in this study, study coordinator, the clinical investigators and support staff.
Funding
This study was supported by scientific research project of Jiangsu Medical Products Administration (202106).
Author information
Authors and Affiliations
Contributions
Designed Research: Wei Qian, Hui-ping wang. Performed Research : Ming-min Cai, Ting Dou, Lu Tang, Qiu-yue Sun. Analyzed Data: Ming-min Cai, Zi-hong Zhai. Wrote Manuscript: Ming-min Cai, Wei Qian. Language Modification:Ming-min Cai, Wei Qian.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in the study involving human participants were conducted in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Consent of publication was obtained from all authors.
Conflict of interest
The author reports no conflicts of interest in this work.
Disclosure of potential conflicts of interest
The author reports no conflicts of interest in this work.
Research involving Human Participants and/or Animals
The phase I clinical trial was approved by Ethics Committee.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cai, Mm., Dou, T., Tang, L. et al. Effects of rifampicin on antineoplastic drug pyrotinib maleate pharmacokinetics in healthy subjects. Invest New Drugs 40, 756–761 (2022). https://doi.org/10.1007/s10637-022-01241-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01241-7