Summary
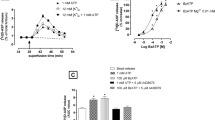
The effects of 5-hydroxytryptamine (5-HT) and of a number of 5-HT receptor agonists and antagonists on the release of endogenous aspartate were investigated in rat cerebellum slices and synaptosomes depolarized with high K+. The release of endogenous aspartate evoked from slices by 35 mmol/l KCl and from synaptosomes by 15 mmol/1 KCl was strongly (about 90%) calcium-dependent. In slices the release of aspartate was inhibited by exogenous 5-HT (0.1–100 nmol/1) in a concentration-dependent manner. The indoleamine was very potent, producing 30% inhibition at 0.1 nmol/l. The effect of 10 nmol/1 5-HT was partly but maximally counteracted by ketanserin (300–1000 nmol/1), a 5-HT2 receptor antagonist, but fully blocked by 300 nmol/1 of the mixed 5-HT1/5-HT2 receptor antagonist methiothepin. The 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT) and the 5-HT2 receptor agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) inhibited the K+-evoked release of endogenous aspartate in a concentration-dependent manner. The effect of 8-OH-DPAT was antagonized by methiothepin, but not by ketanserin which fully antagonized the inhibition produced by DOI. In cerebellar synaptosomes the release of endogenous aspartate evoked by 15 mmol/l K+ was inhibited by exogenous 5-HT and by 8-OH-DPAT, but not by DOI. Methiothepin (100–300 nmol/1) antagonized the inhibitory effects of 100 nmol/l 5-HT or 8-OH-DPAT. However, 1000 nmol/l of various 5-HT receptor antagonists [ketanserin, methysergide, (−)-propranolol, spiperone or ICS 205–930] did not counteract the effect of 100 nmol/15-HT.
It is concluded that: (1) 5-HT projections to the rat cerebellum may exert a potent inhibitory action on the depolarization-evoked release of aspartate; (2) this inhi bition is mediated through receptors of both the 5-HT1 and the 5-HT2 type; (3) the 5-HT2 receptors appear to be sited on structures which do not survive the standard preparation of synaptosomes, while the 5-HT1 receptors are likely to be localized on Apartate-releasing nerve terminals; and (4) the 5-HT1 receptors do not conform to the pharmacological criteria defining the known subtypes of the 5-HT1-binding sites.
Similar content being viewed by others
References
Balcar VJ, Johnston GAR (1972) The structural speciificity of the high affinity uptake of l-glutamate and l-aspartate by rat brain slices. J Neurochem 19:2657–2666
Barnes S, Leighton GE, Davies JA (1988) A novel superfusion chamber for the measurement of endogenous glutamate release from cerebellar slices. J Neurosci Methods 23:57–61
Beitz AJ, Larson AA, Monaghan P, Altschuler RA, Mullett MM, Madl JE (1986) Immunohistochemical localization of glutamate, glutaminase and aspartate aminotransferase in neurons of the pontine nuclei of the rat. Neuroscience 17:741–753
Bonanno G, Pellegrini G, Asaro D, Fontana G, Raiteri M (1989) GABAB autoreceptors in rat cortex synaptosomes: response under different depolarizing and ionic conditions. Eur J Pharmacol [Mol Pharmacol] 172:41–49
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:246–254
Chesselet MF (1984) Presynaptic regulation of neurotransmitter release in the brain: Facts and hypothesis. Neuroscience 12:347–375
Corradetti R, Moneti G, Moroni F, Pepeu G, Wierazsko A (1983) Electrical stimulation of the stratum radiatum increases the release and neosynthesis of aspartate, glutamate and γ-aminobutyric acid in rat hippocampal slices. J Neurochem 41:1516–1525
Docherty M, Bradford HF, Wu J-Y (1987) Co-release of glutamate and aspartate from cholinergic and GABAergic synaptosomes. Nature 330:64–66
Flint RS, Rea MA, McBride WJ (1981) In vitro release of endogenous amino acids from granule cell-, stellate cell-, and climbing fiber-deficient cerebella. J Neurochem 37:1425–1430
Fonnum F, Paulsen RH, Fosse VM, Engelsen B (1986) Synthesis and release of amino acid transmitters. In: Schwarcz R, BenAri Y (eds) Excitatory amino acids and epilepsy. Plenum Press, New York, pp 285–293
Fonnum F, Fykse EM, Paulsen R (1988) Excitatory amino acids: physiology, anatomy and biochemistry. In: Zimmermann H (ed) Cellular and molecular basis of synaptic transmission. Springer, Berlin Heidelberg New York, pp 171–183
Fozard JR (1987) 5-HT: The enigma variations. Trends Pharmacol Sci 8:501–506
Freeman AR, Shank RP, Kephart J, Dekin M, Wang M (1981) A model for excitatory transmission at a glutamate synapse. In: Di Chiara G, Gessa GL (eds) Raven Press, New York, pp 227–243
Girault JA, Barbeito L, Spampinato U, Gozlan H, Glowinski J, Besson MJ (1986) In vivo release of endogenous amino acids from the rat striatum: Further evidence for a role of glutamate and aspartate in corticostriatal neurotransmission. J Neurochem 47: 98–106
Gray EG, Whittaker VP (1962) The isolation of nerve endings from brain: An electron microscopic study of cell fragments derived by homogenization and centrifugation? J Anat 96:79–87
Herrick-Davis K, Titeler M (1988) Detection and characterization of the serotonin 5-HT1D receptor in rat and human brain. J Neurochem 50:1624–1631
Heuring RE, Peroutka SJ (1987) Characterization of a novel 3H-5-hydroxytryptamine binding site subtype in bovine brain membranes. J Neurosci 7:894–903
Israel M, Whittaker VP (1965) The isolation of mossy fibre endings from the granular layer of the cerebellar cortex. Experientia XXI:325–326
Langer SZ (1981) Presynaptic regulation of the release of catecholamines. Pharmacol Rev 32:337–362
Levi G, Gordon RD, Gallo V, Wilkin GP, Balazs R (1982) Putative acidic amino acid transmitters in the cerebellum: I. Depolarization-induced release. Brain Res 239:425–445
Logan WJ, Snyder SH (1972) High affinity uptake systems for glycine, glutamate and aspartate synaptosomes of rat central tissues. Brain Res 42:413–431
Marchi M, Raiteri M (1989) Interaction acetylcholine-glutamate in rat hippocampus: involvement of two subtypes of M-2 muscarinic receptors. J Pharmacol Exp Ther 248:1255–1260
Maura G, Roccatagliata E, Ulivi M, Raiteri M (1988a) Serotonin-glutamate interaction in rat cerebellum: involvement of 5-HT1 and 5-HT2 receptors. Eur J Pharmacol 145:31–38
Maura G, Giardi A, Raiteri M (1988b) Release-regulating D-2 dopamine receptors are located on striatal glutamatergic nerve terminals. J Pharmacol Exp Ther 247:680–684
Maura G, Carbone R, Raiteri M (1989) Aspartate-releasing nerve terminals in rat striatum possess D-2 dopamine receptors mediating inhibition of release. J Pharmacol Exp Ther 251:1142–1146
McMahon HT, Nicholls DG (1990) Glutamine and aspartate loading of synaptosomes: a reevaluation of effects on calcium-dependent excitatory amino acid release. J Neurochem 54: 373–380
Middlemiss DN, Fozard JR (1983) 8-hydroxy-2-(di-n-propyl-amino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur J Pharmacol 90:151–153
Nadler JV, Vaca KW, Frost White W, Lynch GS, Cotman CW (1976) Aspartate and glutamate as possible transmitters of excitatory hippocampal afferents. Nature 260:538–540
Pazos A, Hoyer D, Palacios JM (1984) The binding of serotonergic ligands to the porcine choroid plexus: Characterization of a new type of serotonin recognition site. Eur J Pharmacol 106:539–546
Peroutka SJ, Snyder SH (1981) Two distinct serotonin receptors: Regional variations in receptor binding in mammalian brain. Brain Res 208:339–347
Peroutka SJ (1988) 5-hydroxytryptamine receptor subtypes: molecular, biochemical and physiological characterization. Trends Neurosci 11:496–500
Raiteri M, Angelini F, Levi G (1974) A simple apparatus for studying the release of neurotransmitters from synaptosomes. Eur J Pharmacol 25:411–414
Raiteri M, Marchi M, Maura G (1984) Release of catecholamines and serotonin in synaptosomes. In: Lajtha A (ed) Handbook of neurochemistry, vol 6. Plenum, New York, pp 431–462
Raiteri M, Maura G, Bonanno G, Pittaluga A (1986) Differential pharmacology and function of two 5-HT, receptors modulating transmitter release in rat cerebellum. J Pharmacol Exp Ther 237:644–649
Raiteri M, Marchi M, Costi A, Volpe G (1990) Endogenous aspartate release in the rat hippocampus is inhibited by M2 “cardiacr” muscarinic receptors. Eur J Pharmacol 177:181–187
Richardson BP, Engel G, Donatsch P, Stadler PA (1985) Identification of serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature 316:126–131
Sandoval ME, Cotman CW (1978) Evaluation of glutamate as a neurotransmitter of cerebellar parallel fibers. Neuroscience 3:199–206
Scholfield CN, Moroni F, Corradetti R, Pepeu G (1983) Levels and synthesis of glutamate and aspartate in the olfactory cortex following bulbectomy. J Neurochem 41:135–138
Shannon M, Battaglia G, Glennon RA, Titeler M (1984) 5-HT1 and 5-HT2 binding properties of derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane (2,5-DMA). Eur J Pharmacol 102:23–29
Sills MA, Wolfe BB, Frazer A (1984) Determination of selective and nonselective compounds for the 5-HT1A and 5-HT1B receptor subtypes in rat frontal cortex. J Pharmacol Exp Ther 231:480–487
Starke K, Göthert M, Kilbinger H (1989) Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev 69:864–989
Szerb JC (1988) Changes in the relative amounts of aspartate and glutamate released and retained in hippocampal slices during depolarization. J Neurochem 50:219–224
Tonnaer JADM, Engels GMH, Wiegant VM, Burbach JPH, De Jong W, De Wied D (1983) Proteolytic conversion of angiotensins in rat brain tissue. Eur J Biochem 131:415–421
Tricklebank MD, Forler C, Fozard JR (1984) The involvement of subtypes of the 5-HT1 receptor and of catecholaminergic system in the behavioural response to 8-hydroxy-2-(di-n-propyl-amino)tetralin in the rat. Eur J Pharmacol 106:271–282
Wenthold RJ (1979) Release of endogenous glutamic acid, aspartic acid and GABA from cochlear nucleus slices. Brain Res 162:336–343
Wheeler DD (1984) Kinetics of d-aspartic acid release from rat cortical synaptosomes. Neurochem Res 9:1599–1614
Wiklund L, Bjorklund A, Sjolund B (1977) The indoleaminergic innervation of the inferior olive: I. Convergence with the direct spinal afferents in the areas projecting to the cerebellar anterior lobe. Brain Res 131:1–21
Wiklund L, Toggenburger G, Cuenod M (1982) Aspartate: possible neurotransmitter in cerebellar climbing fibers. Science 216:78–80
Wilkinson R, Nicholls DG (1988) The compartmentation of Ca-dependent pools of glutamate and aspartate in isolated cerebral cortical nerve terminals by isotope exchange and fractionation. Biochem Soc Trans 16:879–880
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maura, G., Barzizza, A., Folghera, S. et al. Release of endogenous aspartate from rat cerebellum slices and synaptosomes: inhibition mediated by a 5-HT2 receptor and by a 5-HT1 receptor of a possibly novel subtype. Naunyn-Schmiedeberg's Arch Pharmacol 343, 229–236 (1991). https://doi.org/10.1007/BF00251120
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00251120