Summary
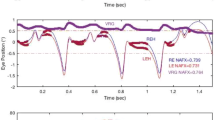
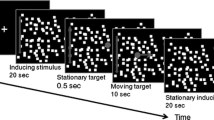
The vertical optokinetic nystagmus (OKN) of 10 normal subjects and the optokinetic afternystagmus (OKAN) of 3 subjects were measured with the magnetic search coil technique. In order to assess the relative contributions of various retinal areas to the up-down asymmetry in OKN the central and peripheral visual fields were selectively stimulated in four OKN conditions. In the full-field OKN condition the stimulus was a 61°×64° display of moving random-dots. Overall, full-field OKN gains elicited by upward motion were significantly higher than those elicited by downward motion at stimulus velocities between 30 and 70°/s. In the periphery-only OKN condition a 3° or 6°-wide vertical band occluded the center of the full-field display. Nine of the 10 subjects displayed OKN in this condition. For 6 subjects, the addition of the 6° band to the full field resulted in an increase in the up-down asymmetry at stimulus velocities above 30°/s. For the other three subjects there was a decline in the gains of both upward and downward OKN when the 3° or 6° band was present; the result was directionally symmetric OKN gains. In the central-strip OKN condition only a 6°-wide central vertical strip of moving dots was visible. The gains of central-strip OKN were not significantly different from the full-field responses. A servo controlled centrally-located 10°× 6° moving display was used in the center-only OKN condition. In this condition both upward and downward gains were attenuated and there was no up-down asymmetry. OKAN was measured following a 50-s exposure to either the full-field or center-only OKN display. The stimulus velocity was 30°/s. After viewing the full-field display the 3 subjects displayed OKAN with slow phases upward following upward OKN but there was no downward OKAN following downward OKN. In contrast, there was no consistent directional asymmetry following exposure to the center-only display. The disappearance of the upward preponderance in OKN and OKAN with occlusion of the peripheral retina suggests that the directional asymmetry in vertical OKN exists in the slow OKN system.
Similar content being viewed by others
References
Abadi RV, Dickinson CM (1985) The influence of preexisting oscillations on the binocular optokinetic response. Ann Neurol 17:578–586
Baloh RW, Honrubia V, Yee RD, Jacobson K (1986) Vertical visual-vestibular interaction in normal human subjects. Exp Brain Res 64:400–406
Baloh RW, Richman L, Yee RD, Honrubia V (1983) The dynamics of vertical eye movements in normal human subjects. Aviat Space Environ Med 54:32–38
Benson AJ, Guedry Jr FE (1971) Comparison of tracking-task performance and nystagmus during sinusoidal oscillation in yaw and pitch. Aerospace Med 42:593–601
Büttner U, Meienberg O, Schimmelpfennig B (1983) The effect of central retinal lesions on optokinetic nystagmus in the monkey. Exp Brain Res 52:248–256
Collewijn H (1969) Optokinetic eye movements in the rabbit: input-output relations. Vision Res 9:117–132
Collewijn H (1972) Latency and gain of the rabbit's optokinetic reactions to small movements. Brain Res 36:59–70
Collewijn H, Tamminga EP (1986) Human fixation and pursuit in normal and open-loop conditions: effects of central and peripheral retinal targets. J Physiol 379:109–129
Cohen B, Matsuo V, Raphan T (1977) Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol 270:321–344
Cohen B, Henn V, Raphan T, Dennett D (1981) Velocity storage, nystagmus, and visual-vestibular interactions in humans. Ann NY Acad Sci 374:421–433
Dubois MFW, Collewijn H (1979) The optokinetic reactions of the rabbit: relation to the visual streak. Vision Res 19:9–17
Erickson RG, Barmack NH (1980) A comparison of the horizontal and vertical optokinetic reflexes of the rabbit. Exp Brain Res 40:448–456
Farmer SG, Rodieck RW (1982) Ganglion cells of the cat accessory optic system: Morphology and retinal topography. J Comp Neurol 205:190–198
Ford A (1976) Significance of terminal transients in electrooculographic recordings. Arch Ophthalmol 87:899–906
Grasse KL, Cynader MS (1984) Electrophysiology of lateral and dorsal terminal nuclei of the accessory optic system. J Neurophysiol 51:276–292
Grasse KL, Cynader MS (1986) Response properties of single units in the accessory optic system of the dark-reared cat. Dev Brain Res 27:199–210
Grasse KL, Cynader MS (1988) The effect of visual cortex lesions on vertical optokinetic nystagmus in the cat. Brain Res, in press
Grasse KL, Cynader MS, Douglas RM (1984) Alterations in response properties in the lateral and dorsal terminal nuclei of the cat accessory optic system following visual cortex lesions. Exp Brain Res 55:69–80
Hainline L, Lemerise E, Abramov I, Turkel J (1984) Orientational asymmetries in small-field optokinetic nystagmus in human infants. Beh Brain Res 13:217–230
Howard IP, Gonzalez E (1987) Human optokinetic nystagmus in response to moving binocularly disparate stimuli. Vision Res 27:1807–1816
Lisberger SG, Miles FA, Optican LM, Eighmy BB (1981) Optokinetic response in monkey: underlying mechanisms and their sensitivity to long-term adaptive changes in vestibuloocular reflex. J Neurophysiol 45:869–890
Matsuo V, Cohen B (1984) Vertical optokinetic nystagmus and vestibular nystagmus in the monkey: up-down asymmetry and effects of gravity. Exp Brain Res 53:197–216
Matsuo V, Cohen B, Theodore R, de Jong V, Henn V (1979) Asymmetric velocity storage for upward and downward nystagmus. Brain Res 176:159–164
Murasugi CM, Howard IP, Ohmi M (1986) Optokinetic nystagmus: the effects of stationary edges, alone, and in combination with central occlusion. Vision Res 26:1155–1162
Murasugi CM, Howard IP (1989) Human horizontal optokinetic nystagmus elicited by the upper versus the lower visual fields. Vision Neurosci 2:73–79
Muratore R, Zee DS (1979) Pursuit after-nystagmus. Vision Res 19:1057–1059
Mustari MJ, Fuchs AF, Langer TP, Kaneko CRS, Wallman J (1988) The role of the primate lateral terminal nucleus in visuomotor behavior. Progr in Brain Res 75:121–128
Pasik P, Pasik T, Valciukas JA, Bender MB (1971) Vertical optokinetic nystagmus in the split-brain monkey. Exp Neurol 30:162–171
Robinson DA (1963) A method of measuring eye movements using a scleral search coil in a magnetic field. IEEE Trans Biomed Electr 10:137
Schor CM, Levi DM (1980) Disturbances of small-field horizontal and vertical optokinetic nystagmus in amblyopia. Invest Ophthal Vis Sci 19:668–683
Simpson JI, Soodak RE, Hess R (1979) The accessory optic system and its relation to the vestibulocerebellum. Progr in Brain Research 50:715–724
Skrandies W (1987) The upper and lower visual field of man: electrophysiological and functional differences. Prog Sens Physiol 8:1–93
ter Braak JWG (1936) Untersuchungen über optokinetischen Nystagmus. Arch Néelandaises Physiol l'homme animaux 21:309–376
Van den Berg AV, Collewijn H (1988) Directional asymmetries of human optokinetic nystagmus. Exp Brain Res 70:597–604
van Die GC, Collewijn H (1982) Optokinetic nystagmus in man: role of central and peripheral retina and occurrence of asymmetries. Human Neurobiol 1:111–119
van Die GC, Collewijn H (1986) Control of human optokinetic nystagmus by the central and peripheral retina: effects of partial visual field masking, scotopic vision and central retinal scotomata. Brain Res 383:185–194
Winterson BJ, Steinman RM (1978) The effect of luminance on human smooth pursuit of perifoveal and foveal targets. Vision Res 18:1165–1172
Yee RD, Schiller VL, Lim V, Baloh FG, Baloh RW, Honrubia V (1985) Velocities of vertical saccades with different eye movement recording methods. Invest Ophthal Vis Sci 26:938–944
Zee DS, Yamazaki A, Butler PH, Güncer G (1981) Effects of ablation of flocculus and paraflocculus on eye movements in primate. J Neurophysiol 46:878–899
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murasugi, C.M., Howard, I.P. Up-down asymmetry in human vertical optokinetic nystagmus and afternystagmus: contributions of the central and peripheral retinae. Exp Brain Res 77, 183–192 (1989). https://doi.org/10.1007/BF00250580
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00250580