Abstract
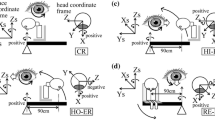
We investigated the effects of pursuit effort against the optokinetic nystagmus (OKN) on induced motion (IM) by measuring vertical IM and eye movements. Participants viewed an inducing stimulus (a random dot pattern) moving either upward or downward at the velocity of 10 or 40 °/s. A horizontally moving target (a single dot) was then presented within the inducing stimulus. Participants were asked to pursue the target and report the perceived slant of the target motion path by using a joystick. The results showed that IM magnitude was larger with an upward stimulation than with a downward stimulation. IM magnitude was also larger at 40 °/s than at 10 °/s. The results of eye movements prior to the target presentation showed that OKN was elicited more effectively with an upward stimulation than with a downward stimulation and at 40 °/s than at 10 °/s. OKN was markedly reduced when the target was presented within the inducing stimulus. These results support the oculomotor theory that IM magnitude reflects pursuit effort against OKN in response to an inducing stimulus.








Similar content being viewed by others
Notes
Because we used a flat screen, the velocity would be slightly slower at the periphery of the screen.
This value is the manufacturer’s specification based on measurements by using an artificial eye with pupil size of 4 mm. In the present study, the mean pupil size of participants during the task was 5.82 mm (SD = 0.97 mm). We contacted the company and confirmed that the spatial resolution in the present study was less than 0.1°.
We also conducted correlation analyses with other eye movement measures and found that the correlations were quite weak; the mean correlation coefficient over 11 participants was .194, .299, .194, and −.070 for standard deviation, amplitude, number of quick-phase saccades, and horizontal pursuit velocity, respectively.
References
Bahill, A. T., & LaRitz, T. (1984). Why can't batters keep their eyes on the ball? American Scientist, 72, 249–253.
Brosgole, L. (1966). Change in phenomenal location and perception of motion. Perceptual & Motor Skills, 23, 999–1001.
Brosgole, L. (1968). An analysis of induced motion. Acta Psychologica, 28, 1–44.
Cohen, B., Matsuo, V., & Raphan, T. (1977). Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. Journal of Physiology, 270, 321–344.
Collins, W. E., Schroeder, D. J., Rice, N., Mertens, R. A., & Kranz, G. (1970). Some characteristics of optokinetic eye movement patterns: A comparative study. Aerospace Medicine, 41, 1251–1262.
Di Russo, F., Pitzalis, S., & Spinelli, D. (2003). Fixation stability and saccadic latency in elite shooters. Vision Research, 43, 1837–1845.
Duncker, K. (1929/1938). Über induyierte Bewegung (Ein Beitrag zur Theorie optisch wahrgenommener Bewegung). In W. D. Ellis (Ed. And Trans.), Source book of Gestalt psychology (pp. 161-172). London; Routledge & Kegan Paul. (Reprinted from Psychologische Forschung, 1929, 12, 180–259)
Farrell-Whelan, M., Wenderoth, P., & Wiese, M. (2012). Studies of the angular function of a Duncker-type induced motion illusion. Perception, 41, 733–746.
Garbutt, S., Han, Y., Kumar, A. N., Harwood, M., Harris, C. M., & Leigh, R. J. (2003). Vertical optokinetic nystagmus and saccades in normal human subjects. Investigative Ophthalmology & Visual Science, 44, 3833–3841.
Gogel, W. C. (1977). Independent motion induction in separated portions of the visual field. Bulletin of the Psychonomic Society, 10, 408–410.
Gogel, W. C., & Griffin, B. W. (1982). Spatial induction of illusory motion. Perception, 11, 187–199.
Gogel, W. C., & Koslow, M. (1972). The adjacency principle and induced movement. Perception & Psychophysics, 11, 309–314.
Gogel, W. C., & MacCracken, P. J. (1979). Depth adjacency and induced motion. Perceptual & Motor Skills, 48, 343–350.
Heckmann, T., & Post, R. B. (1988). Induced motion and optokinetic aftemystagmus: Parallel response dynamics with prolonged stimulation. Vision Research, 28, 681–694.
Ilg, U. J. (1997). Slow eye movements. Progress in Neurobiology, 53, 293–329.
Kawano, K., & Miles, F. A. (1986). Short-latency ocular following responses of monkey. II. Dependence on a prior saccadic eye movement. Journal of Neurophysiology, 56, 1355–1380.
Knapp, C. M., Gottlob, I., McLean, R. J., & Proudlock, F. A. (2008). Horizontal and vertical look and stare optokinetic nystagmus symmetry in health adult volunteers. Investigative Ophthalmology & Visual Science, 49, 581–588.
Knapp, C. M., Proudlock, F. A., & Gottlob, I. (2013). OKN asymmetry in human subjects: A literature review. Strabismus, 21, 37–49.
Krauzlis, R. J. (2004). Recasting the smooth pursuit eye movement system. Journal of Neurophysiology, 91, 591–603.
Krauzlis, R. J., & Miles, F. A. (1996). Decreases in the latency of smooth pursuit and saccadic eye movements produced by the “gap paradigm” in the monkey. Vision Research, 36, 1973–1985.
Land, M. F., & McLeod, P. (2000). From eye movements to actions: how batsmen hit the ball. Nature Neuroscience, 3, 1340–1345.
Lott, L. A., & Post, R. B. (1993). Up-down asymmetry in vertical induced motion. Perception, 22, 527–535.
Mack, A. (1986). Perceptual aspects of motion in the frontal plane. In K. R. Boff, L. Kaufman, & J. P. Thomas (Eds), Handbook of Perception and Human Performance Vol.1 Sensory processes and perception (Chap 17, pp.1–38). New York; John Wiley & Sons.
Matsuo, V., & Cohen, B. (1984). Vertical optokinetic nystagmus and vestibular nystagmus in the monkey: up-down asymmetry and effects of gravity. Experimental Brain Research, 53, 197–216.
Miles, F. A., & Kawano, K. (1986). Short-latency ocular following responses of monkey. III. Plasticity. Journal of Neurophysiology, 56, 1381–1396.
Miles, F. A., Kawano, K., & Optican, L. M. (1986). Short-latency ocular following responses of monkey. I. Dependence on temporospatial properties of the visual input. Journal of Neurophysiology, 56, 1321–1354.
Murasugi, C. M., & Howard, I. P. (1989). Up-down asymmetry in human vertical optokinetic nystagmus and afternystagmus: contribution of the central and peripheral retinae. Experimental Brain Research, 77, 183–192.
Nakayama, K., & Tyler, C. W. (1978). Relative motion induced between stationary lines. Vision Research, 18, 1663–1668.
Pola, J., & Wyatt, H. J. (1989). The perception of target motion during smooth pursuit eye movements in the open-loop condition: characteristics of retinal and extraretinal signals. Vision Research, 29, 471–483.
Post, R. B. (1986). Induced motion considered as a visually induced oculogyral illusion. Perception, 15, 13l–138l.
Post, R. B., Chi, D., Heckmann, T., & Chaderjian, M. (1989). A reevaluation of the effect of velocity on induced motion. Perception & Psychophysics, 45, 411–416.
Post, R. B., & Heckmann, T. (1986). Induced motion and apparent straight ahead during prolonged stimulation. Perception & Psychophysics, 40, 263–270.
Post, R. B., & Leibowitz, H. W. (1985). A revised analysis of the role of efference in motion perception. Perception, 14, 631–643.
Post, R. B., & Lott, L. A. (1990). Relationship of induced motion and apparent straight-ahead shifts to optokinetic stimulus velocity. Perception & Psychophysics, 48, 401–406.
Post, R. B., Shupert, C. L., & Leibowitz, H. W. (1984). Implications of OKN suppression by smooth pursuit for induced motion. Perception & Psychophysics, 36, 493–498.
Reinhardt-Rutland, A. H. (1988). Induced movement in the visual modality: an overview. Psychological Bulletin, 103, 57–71.
Schor, C., & Narayan, V. (1981). The influence of field size upon the spatial frequency response of optokinetic nystagmus. Vision Research, 21, 985–994.
Seya, Y., & Mori, S. (2007). Motion illusion reveals fixation stability of karate athletes. Visual Cognition, 15, 491–512.
Takahashi, M., Sakurai, S., & Kanzaki, J. (1978). Horizontal and vertical optokinetic nystagmus in man. Otorhinolaryngology, 40, 43–52.
van den Berg, A. V., & Collewijn, H. (1988). Directional asymmetries of human optokinetic nystagmus. Experimental Brain Research, 70, 597–604.
Wallach, H., Bacon, J., & Schulman, P. (1978). Adaptation in motion perception: Alteration of induced motion. Perception & Psychophysics, 24, 509–514.
Zivotofsky, A. Z. (2004). The Duncker illusion: Intersubject variability, brief exposure, and the role of eye movements in its generation”. Investigative Ophthalmology & Visual Science, 45, 2867–2872.
Author note
This research was supported by Grants-in-Aid for Young Scientists (B) 25870916 to Y.S. and for Scientific Research (B) 21300230 to K.I.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Results of an additional experiment in which 10 new participants (8 males and 2 females; mean age = 23.0 years, range = 21–25 years) were presented with stimuli identical to those used in the present study, except that the dot size was smaller (at 0.45°). a Mean induced motion (IM) magnitude. b Mean slow-phase velocity. c Mean standard deviation of eye position. IS, inducing stimulus. The vertical bars indicate standard errors of the means
For the mean IM magnitude, a two-way ANOVA showed significant main effects of velocity, F(1, 9) = 6.72, η p 2 = .427, p = .029, and direction, F(1, 9) = 6.30, η p 2 = .412, p = .033, and a significant interaction between velocity and direction, F(1, 9) = 7.56, η p 2 > .456, p < .023. Simple main effect of velocity was significant with upward stimulation, F(1, 9) = 17.27, η p 2 = .657, p = .003, while it was not with downward stimulation. Simple main effect of direction was significant at 40 °/s, F(1, 9) = 8.50, η p 2 = .486, p = .017, while it was not at 10 °/s.
For the mean slow-phase velocity, a three-way ANOVA showed significant main effects of interval, F(1, 9) = 112.76, η p 2 = .926, p < .001, velocity, F(1, 9) = 79.19, η p 2 = .898, p < .001, and direction, F(1, 9) = 7.92, η p 2 = .468, p = .020. There were significant interactions of interval and velocity, F(1, 9) = 61.00, η p 2 = .871, p < .001, and velocity and direction, F(1, 9) = 7.73, η p 2 = .462, p = .021. Subsequent analyses of interval × velocity revealed a significant simple main effect of velocity before the target onset, F(1, 9) = 76.02, η p 2 = .894, p < .001, but no significant effect of velocity after the onset of the target’s motion. Subsequent analyses of velocity × direction revealed significant differences between the two velocities with upward, F(1, 9) = 63.54, η p 2 = .876, p < .001, and downward, F(1, 9) = 7.71, η p 2 = .461, p = .022, stimulations. There were also significant differences between the two directions at 10 °/s, F(1, 9) = 6.34, η p 2 = .413, p = .033, and 40 °/s, F(1, 9) = 7.99, η p 2 = .470, p = .020.
For the standard deviation, a three-way ANOVA showed that all the main effects and interactions were significant [interval, F(1, 9) = 49.28, η p 2 = .846, p < .001; velocity, F(1, 9) = 54.88, η p 2 = .859, p < .001; direction, F(1, 9) = 23.78, η p 2 = .725, p = .001; interval × velocity, F(1, 9) = 17.69, η p 2 = .663, p = .002; interval × direction, F(1, 9) = 18.78, η p 2 = .676, p = .002; velocity × direction, F(1, 9) = 29.21, η p 2 = .764, p < .001; interval × velocity × direction, F(1, 9) = 23.77, η p 2 = .725, p = .001]. Separate ANOVAs showed significant main effects of velocity, F(1, 9) = 34.23, η p 2 = .792, p < .001, and direction, F(1, 9) = 35.25, η p 2 = .797, p < .001, and a significant interaction between velocity and direction, F(1, 9) = 42.31, η p 2 = .825, p < .001. Subsequent analyses showed significant differences between the two velocities with upward, F(1, 9) = 69.76, η p 2 = .886, p < .001, and downward, F(1, 9) = 7.24, η p 2 = .446, p = .025, stimulations. There were also significant differences between the two directions at 10 °/s, F(1, 9) = 11.18, η p 2 = .554, p = .008, and 40 °/s, F(1, 9) = 43.48, η p 2 = .828, p < .001. No effect or interaction was significant after the initiation of target stimulus motion.
Rights and permissions
About this article
Cite this article
Seya, Y., Ishihara, M. & Imanaka, K. Up–down asymmetry in vertical induced motion and optokinetic nystagmus. Atten Percept Psychophys 77, 220–233 (2015). https://doi.org/10.3758/s13414-014-0734-z
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-014-0734-z