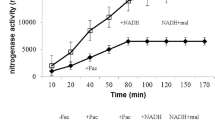
Abstract
Rhodobacter capsulatus strains E1F1 and B10 and Rhodobacter sphaeroides DSM 158 did not use hydroxylamine as nitrogen source for growth but metabolized it mainly through the glutamine synthetase reaction. Hydroxylamine had a high toxicity for cells growing either under phototrophic or dark-aerobic conditions. l-methionine-d,l-sulfoximine partially inhibited hydroxylamine uptake and increased the inhibition time of nitrogenase activity by this nitrogen compound. Nitric oxide was also a powerful inhibitor of nitrogenase in intact cells of R. capsulatus. Since low amounts of NO were produced from hydroxylamine, short-term inhibition of nitrogenase in the presence of this compound could be mediated in vivo by nitric oxide.
Similar content being viewed by others
Abbreviations
- GS:
-
glutamine synthetase
- MSX:
-
l-methionine-d,l-sulfoximine
- MTA:
-
mixed alkyltrimethylammonium bromide
References
Aebi HE (1983) Catalase. In: Bergmeyer HU, Bergmeyer J, Grassl M (eds) Methods of enzymatic analysis, vol. III. Verlag Chemie, Weinheim, pp 273–286
Arp DJ, Zumft WG (1983) l-methionine-d,l-sulfoximine as a probe for the role of glutamine synthetase in nitrogenase switch-off by ammonia and glutamine in Rhodopseudomonas palustris. Arch Microbiol 134: 17–22
Balandin T, Fernández VM, Aparicio PJ (1986) Characterization of the reversible inactivation of Ankistrodesmus braunii nitrate reductase by hydroxylamine. Plant Physiol 82: 65–70
Caballero FJ, Cejudo FJ, Florencio FJ, Cárdenas J, Castillo F (1985) Molecular and regulatory properties of glutamine synthetase from the phototrophic bacterium Rhodopseudomonas capsulata E1F1. J Bacteriol 162: 804–809
Cammack R, Fry IV, Payne MJ (1987) The significance of iron-nitrosyl complexes in biology and in the reaction of assimilatory nitrite reductase. In: Ullrich WR, Aparicio PJ, Syrett PJ, Castillo F (eds) Inorganic nitrogen metabolism. Springer, Berlin Heidelberg New York, pp 192–194
Castillo F, Cárdenas J (1982) Nitrite inhibition of bacterial dinitrogen fixation. Z Naturforsch 37: 784–786
Castillo F, Caballero FJ, Cárdenas J (1981) Nitrate photoassimilation by the phototrophic bacterium Rhodopseudomonas capsulata E1F1. Z Naturforsch 36: 1025–1029
DeMaster EG, Raij L, Archer SL, Weir EK (1989) Hydroxylamine is a vasorelaxant and a possible intermediate in the oxidative conversion of l-arginine to nitric oxide. Biochem Biophys Res Commun 163: 527–533
Hewitt EJ, Nicholas DJD (1964) Enzymes of inorganic nitrogen metabolism. In: Peach K, Tracey MV (eds) Modern methods of plant analysis. Springer, Berlin Heidelberg New York, pp 67–172
Jones BL, Monty KJ (1979) Glutamine as a feedback inhibitor of the Rhodopseudomonas sphaeroides nitrogenase system. J Bacteriol 139: 1007–1013
Jouanneau Y, Meyer CM, Vignais PM (1983) Regulation of nitrogenase activity through iron protein interconversion into an active and an inactive form in Rhodopseudomonas capsulata. Biochim Biophys Acta 749: 318–328
Jouanneau Y, Roby C, Meyer C, Vignais PM (1989) ADP-ribosylation of dinitrogenase reductase in Rhodobacter capsulatus. Biochemistry 28: 6524–6530
Keilin D, Nicholls P (1958) Reactions of catalase with hydrogen peroxide and hydrogen donors. Biochim Biophys Acta 29: 302–307
Kerber NL, Cárdenas J (1982) Nitrate reductase from Rhodopseudomonas sphaeroides. J Bacteriol 150: 1091–1097
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
Ludden PW, Burris RH (1976) Activating factor for the iron protein of nitrogenase from Rhodospirillum rubrum. Science 194: 424–426
Ludden PW, Roberts GP, Lowery RG, Fitzmaurice WP, Saari LL, Lehman L, Lies D, Woehle D, Wirt H, Murrel SA, Pope MR, Kanemoto RH (1988) Regulation of nitrogenase activity by reversible ADP-ribosylation of dinitrogenase reductase. In: Bothe H, Bruijn FJde, Newton WE (eds) Nitrogen fixation: hundred years after. Fischer, Stuttgart, pp 157–162
Martínez M, Castillo F (1991) Inhibition of aconitase and fumarase by nitrogen compounds in Rhodobacter capsulatus. Arch Microbiol 155: 149–152
Meyer J (1981) Comparison of carbon monoxide, nitric oxide, and nitrite as inhibitors of the nitrogenase from Clostridium pasteurianum. Arch Biochem Biophys 210: 246–256
Michalski WP, Nicholas DJD (1987) Inhibition of nitrogenase by nitrite and nitric oxide in Rhodopseudomonas sphaeroides f. sp. denitrificans. Arch Microbiol 147: 304–308
Moreno-Vivián C, Cárdenas J, Castillo F (1986) In vivo short-term inhibition of nitrogenase by nitrate in Rhodopseudomonas capsulata E1F1. FEMS Microbiol Lett 34: 105–109
Moreno-Vivián C, Caballero FJ, Cárdenas J, Castillo F (1989) Effect of the C/N balance on the regulation of nitrogen fixation in Rhodobacter capsulatus E1F1. Biochim Biophys Acta 977: 297–300
Prusiner S (1973) Glutaminases of Escherichia coli: properties, regulation and evolution. In: Prusiner S, Stadtman ER (eds) The enzymes of glutamine metabolism. Academic Press, New York, pp 293–316
Rigaud J, Bergersen FJ, Turner GL, Daniel RM (1973) Nitrate dependent acetylene-reduction and nitrogen-fixation by soybean bacteroids. J Gen Microbiol 77: 137–144
Scolnik PA, Virosco J, Haselkorn R (1983) The wild-type gene for glutamine synthetase restores ammonia control of nitrogen fixation to Gln- (glnA) mutants of Rhodopseudomonas capsulata. J Bacteriol 155: 180–185
Shapiro BM, Stadtman ER (1970) Glutamine synthetase (Escherichia coli). In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol. 17A. Academic Press, London, pp 115–124
Stewart WDP, Fitzgerald GP, Burris RH (1968) Acetylene reduction by nitrogen-fixing blue-green algae. Arch Mikrobiol 62: 336–348
Tsang DC, Suzuki I (1982) Cytochrome c-554 as a possible electron donor in the hydroxylation of ammonia and carbon monoxide in Nitrosomonas europaea. Can J Biochem 60: 1018–1024
Vega JM, Kamin H (1977) Spinach nitrite reductase. Purification and properties of a siroheme-containing iron-sulfur enzyme. J Biol Chem 252: 896–909
Weaver PF, Wall JD, Gest H (1975) Characterization of Rhodopseudomonas capsulata. Arch Microbiol 136: 147–151
Yoshikawa S, Orii Y (1970) The inhibition mechanism of the cytochrome oxydase reaction. I. Inhibition by hydroxylamine. J Biochem 68: 145–156
Zumft WG, Castillo F (1978) Regulatory properties of the nitrogenase from Rhodopseudomonas palustris. Arch Microbiol 117: 53–60
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Caballero, F.J., Igeño, M.I., Quiles, R. et al. In vivo inhibition of nitrogenase by hydroxylamine in Rhodospirillaceae Role of nitric oxide. Arch. Microbiol. 158, 14–18 (1992). https://doi.org/10.1007/BF00249059
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00249059