Abstract
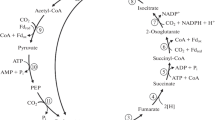
Autotrophically grown cells of Chloroflexus aurantiacus B-3 were shown to possess activity of ATP-dependent malate lyase (acetylating CoA). ATP: malate lyase is supposed to be the specific enzyme of the cycle of the autotrophic CO2 fixation, in which pyruvate synthase, pyruvate phosphate dikinase, phosphoenolpyruvate (PEP) carboxylase and malate dehydrogenase are involved as well. The main product of the CO2 fixation cycle is glyoxylate, which could further be converted into 3-phosphoglyceric acid (3-PGA) in the reactions of either glycerate or serine pathway. The enzymes of both pathways were detected in C. auratiacus B-3. The results of the in vivo studies of glyxoylate and glycine metabolism, as well as the inhibitor analysis using fluoroacetate (FAc), isonicotinic acid hydrazide (INH), and 4-aminopterin (4-AP) confirm the operation of the proposed pathway in Chloroflexus.
Similar content being viewed by others
Abbreviations
- 3-PGA:
-
3-phosphoglyceric acid
- 4-AP:
-
4-aminopterin
- FAc:
-
fluoroacetate
- INH:
-
isonicotinic acid hydrazide
- MV:
-
methyl viologen
- PEP:
-
phosphoenolpyruvate
- THF:
-
tetrahydrofolate
- TPP:
-
thiamine pyrophosphate
References
Albers H, Gottschalk G (1976) Acetate metabolism in Rhodopseudomonas gelatinosa and several other Rhodospirillaceae. Arch Microbiol 111: 45–49
Bassham JA, Buchanan BB (1982) Pathway of CO2 fixation in plants and bacteria. In: Govindjee (ed) Photosynthesis, vol 2. Academic Press, New York London, pp 218–272
Baker BR (1967) Design of active-site-directed irreversible enzyme inhibitor. Academic Press. New York London
Blackmore MA, Quayle JR (1970) Microbial growth on oxalate by a route not involving glyoxylate carboligase. Biochem J 118: 53–59
Botsford JL, Parks LW (1969) Serine transhydroxymetylase in methionine biosynthesis in Saccharomyces cereviseae. J Bacteriol 97: 1176–1183
Bradford MM (1976) A rapid and sensitive method for the quantation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Castenholz RW, Pierson BK (1981) Isolation of members of the family Chloroflexaceae. In: Balows A, Trüper HG, Dworkin M et al. (eds) The prokaryotes, vol 1, Springer, Berlin Heidelberg New York, pp 290–298
Codd GA, Stewart WDP (1973) Pathways of glycollate metabolism in the blue-green alga Anabaena cylindrica. Arch Mikrobiol 94: 11–28
Den H, Robinson WG, Coon M (1959) Enzymatic conversion of β-hydroxypropionate to malonate semialdehyde. J Biol Chem 234: 1666–1671
Dixon GH, Kornberg HL (1959) Assay methods for key enzymes of the glyoxylate cycle. Biochem J 72: 195–198
Durham NN, Jacobs CD, Fergusson D (1964) Relationship of a β-alanine-pyruvic aminotransferase to reversal of D-serine inhibition of growth. J Bacteriol 88: 1525–1528
Fuchs G (1989) Alternative pathways of autotrophic CO2 fixation. In: Schlegel HG, Bowien B (eds) Autotrophic bacteria. Science Tech Publishers, Madison, Wis., pp 365–382
Hayashi O, Yatsuoki N, Tatibana M, Takeshita M, Kuno S (1961) Enzymatic studies on the metabolism of β-alanine. J Biol Chem 236: 781–790
Hayashi S, Lin ECC (1967) Purification and properties of glycerate kinase from Escherichia coli. J Biol Chem 242: 1030–1035
Holo H (1989) Chloroflexus aurantiacus secretes 3-hydrohypropionate, a possible intermediate in the assimilation of CO2 and acetate. Arch Microbiol 151: 252–256
Holo H, Grace D (1987) Polyglucose synthesis in Chloroflexus aurantiacus studied by 13C-NMR. (Evidence for acetate metabolism by a new metabolic pathway in autotrophically grown cells). Arch Microbiol 148: 292–297
Holo H, Sirevåg R (1986) Autotrophic growth and CO2 fixation of Chloroflexus aurantiacus. Arch Microbiol 145: 173–180
Kondratieva EN (1979) Interrelation between modes of carbon assimilation and energy production in phototrophic purple and green bacteria. In: Quayle JR (ed) Microbial biochemistry, vol 21. University Park Press, Baltimore, pp 117–175
Kornberg HL, Gotto AM (1961) The metabolism of C2 compounds om microorganisms. VI. Synthesis of cell constituents from glycollate by Pseudomonas sp. Biochem J 78: 69–72
Krakow G, Barkalis SS, Hayashi JA (1961) Glyoxylic acid carboligase: an enzyme present in glycolate-grown Escherichia coli. J Bacteriol 81: 509–518
Krasilnikova EN, Pedan LV, Firsov NN, Kondratieva EN (1973) Enzymes of the citric acid cycle in various species of phototrophic bacteria (in Russian) Mikrobiologia 42: 995–1000
Krasilnikova EN, Keppen OI, Gorlenko VM, Kondratieva EN (1986) Chloroflexus aurantiacus growth in media with different organic compounds and the pathways of their metabolism (in Russian). Mikrobiologia 55: 425–430
Kun E, Gottwald LK, Fanshier DH, Ayling JE (1963) The effects of difluoro-oxaloacetate and difluoromalate on malate dehydrogenases and glutamate-aspartate aminotransferase. J Biol Chem 238: 1456–1463
Laakmann-Ditges G, Klemme J-H (1986) Occurrence of two L-threonine (L-serine) dehydratases in the thermophile Chloroflexus aurantiacus. Arch Microbiol 144: 219–221
Larsen H (1952) On the culture and general physiology of the green sulfur bacteria. J Bacteriol 64: 187–196
Løken Ø, Sirevåg R (1982) Evidence for presence of the glyoxylate cycle in Chloroflexus. Arch Microbiol 132: 276–279
Salem AR, Haking J, Quayle JR (1973) Cleavage of malyl-coenzyme A into acetyl-coenzyme A and glyoxylate by Pseudomonas AM1 and other C1-unit-utilizing bacteria. Biochem J 136: 89–96
Scrimgeour KJ, Huennekens FM (1962) Serine hydroxymethylase. In: Colowick SP, Kaplan ND (eds) Methods in enzymology, vol 5. Academic Press, New York London, pp 838–851
Sirevåg R, Ormerod JG (1970) Carbon dioxide fixation in green sulfur bacteria. Biochem J 120: 399–408
Smith BN, Epstein S (1971) Two categories of 13C/12C ratios of higher plants. Plant Physiol 47: 380–384
Stafford HA, Magaldi A, Vennesland B (1954) The enzymatic reduction of hydroxypyruvic acid in higher plants. J Biol Chem 207: 621–629
Takabe T, Akazawa T (1977) A comparative study of the effect O2 on photosynthetic carbon metabolism by Chlorobium thiosulfatophillum and Chromatium vinosum. Plant Cell Physiol 18: 753–765
Thauer RK, Rupprecht E, Jungermann K (1970) Glyoxylate inhibition of clostridial pyruvate synthase. FEBS Lett 9: 271–273
Tuboi S, Kikuchi G (1962) Enzymatic cleavage of malate to glyoxylate and acetyl-coenzyme A. Biochim Biophys Acta 62: 188–192
Yokota A, Asama K, Kitaoka S (1990) Evidence of reentrance of glycolate carbon into photosynthetic carbon reduction cycle in photosynthesizing Euglena gracilis Z Plant Physiol 94: 388–391
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ivanovsky, R.N., Krasilnikova, E.N. & Fal, Y.I. A pathway of the autotrophic CO2 fixation in Chloroflexus aurantiacus . Arch. Microbiol. 159, 257–264 (1993). https://doi.org/10.1007/BF00248481
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00248481