Abstract
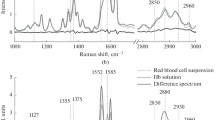
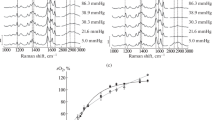
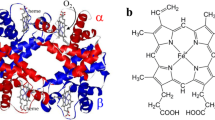
The altered oxygen binding curves for various abnormal hemoglobins were analyzed according to a two-state allosteric model. Of three allosteric parameters computed for abnormal hemoglobins, K R was nearly constant, but K T and L varied with the correlation of log c=−0.4 log L, where c is K R/K T. This correlation indicates that the abnormal allosteric oxygen binding of hemoglobin is due to altered molecular properties of the deoxy-T state but not that of the deoxy-R state. To clarify the molecular basis of this idea, resonance Raman spectra in the low-frequency region of abnormal hemoglobins were measured under different solvent conditions. Varied frequencies of iron-histidine stretching Raman lines was found to correlate with varied oxygen affinities (K T) of deoxy-T states. The strength of the iron-histidine bond of deoxy-T states was changed, depending upon the magnitude of the strain imposed on hemes by globin, and this bond presumably comprises an important part of the regulation mechanisms for hemoglobin oxygen binding and structure changes.
Similar content being viewed by others
References
Argade, P. V., Sassaroli, M., Rousseau, D. L., Inubushi, T., Ikeda-Saito, M., and Lapidot, A. (1984). J. Am. Chem. Soc. 106, 6593–6596.
Bunn, H. F., and Guidotti, G. (1972). J. Biol. Chem. 247, 2345.
Imai, K. (1983). J. Mol. Biol. 167, 741–749.
Kitagawa, T., Nagai, K., and Tsubaki, M. (1979). FEBS Lett. 104, 376–378.
Matsukawa, S., Mawatari, K., Shimokawa, Y., and Yoneyama, Y. (1981). J. Mol. Biol. 150, 615–621.
Matsukawa, S., Mawatari, K., Shimokawa, Y., Takeda, Y., Yoneyama, Y., Itoh, M., Kurokawa, H., and Kitagawa, T. (1985a). Acta Hematol. (Japan) 48, 2002–2014.
Matsukawa, S., Mawatari, K., Yoneyama, Y., and Kitagawa, T. (1985b). J. Am. Chem. Soc. 197, 1108–1113.
Monod, J., Wyman, J., and Changeux, J.-P. (1965). J. Mol. Biol. 12, 88–118.
Nagai, K., Kitagawa, T., and Morimoto, H. (1980). J. Mol. Biol. 136, 271–289.
Ondrias, M. R., Rousseau, D. L., Shelnutt, J. A., and Simon, S. R. (1982). Biochemistry 21, 3428–3437.
Perutz, M. F. (1970). Nature 228, 726–734.
Author information
Authors and Affiliations
Additional information
This article was presented during the proceedings of the International Conference on Macromolecular Structure and Function, held at the National Defence Medical College, Tokorozawa, Japan, December 1985.
Rights and permissions
About this article
Cite this article
Matsukawa, S., Mawatari, K., Yoneyama, Y. et al. Functional and structural analyses on abnormal hemoglobins with impaired oxygen binding properties—To elucidate the allosteric mechanism of hemoglobin. J Protein Chem 6, 109–119 (1987). https://doi.org/10.1007/BF00247760
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00247760