Summary
-
1.
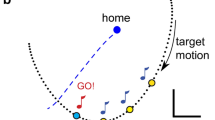
In cats trained to track a moving display by making rapid, isometric force adjustments, responses are characterized by extremely short reaction times (60–70 ms) and a stereotyped temporal configuration. The animal uses early derivatives of display movement to scale force responses to target stimuli of different sizes according to a learned relationship between initial display motion and required force (Ghez and Vicario 1978a, 1978b). In the present study we altered that relationship by using double stimulation and delayed feedback to assess the animals' ability to update their responses.
-
2.
In experiments where a second target stimulus followed the first after a controlled interval (15–120 ms) on random trials, the animal modified its response in the appropriate direction with little or no increase in reaction time. When the second stimulus called for a return to baseline, the animal aborted the ongoing response. When the second stimulus called for a doubling of force, the animal increased its phasic force output; however, this increase was not sufficient to reach the new target level and late responses were emitted.
-
3.
The control response which followed each experimental double stimulation trial showed consistent differences from other controls in the amplitude of both peak force and peak dF/dt. Control responses following trials calling for a return were reduced in size; those following stimuli requiring response doubling were increased. We concluded that the experimental trials not only elicited modification of ongoing responses but also caused the animal to alter its internalized gain function relating initial display derivatives to required force.
-
4.
In experiments where feedback was delayed after giving a first target stimulus such that the compensatory display failed to reflect the animal's initial response, the animal emitted a new updated response 70–80 ms after the first. The display trajectory which caused the cat to update its response on delayed feedback trial was identical to that of control trials with long reaction times. In this case, however, the information eliciting response updating had to be derived as a difference between the actual display trajectory and that expected by the animal, based on its experience with the tracking task. This suggests that the animal develops an internal model of display properties which is used to determine when a new response is required.
Similar content being viewed by others
References
Bahill AT, Clark MR, Stark L (1975) The main sequence, a tool for studying human eye movements. Math Biosci 24: 191–204
Baker R, McCrea RA, Spencer RF (1980) Synaptic organization of cat accessory abducens nucleus. J Neurophysiol 43: 771–791
Baker R, Precht W (1972) Electrophysiological properties of trochlear motoneurons as revealed by IV nerve stimulation. Exp Brain Res 14: 127–157
Brebner J (1968) Continuing and reversing the direction of responding movements: some exceptions to the so-called “psychological refractory period”. J Exp Psychol 78: 120–127
Crutcher MC, DeLong MR (1984) Single cell studies of the primate Putamen. II. Relations to direction of movement and pattern of muscular activity. Exp Brain Res 53: 244–258
Georgopoulos AP, Kalaska JF, Massey JT (1981) Spatial trajectories and reaction times of aimed movements: effects of practice, uncertainty, and change in target location. J Neurophyshiol 46: 725–743
Ghez C (1979) Contributions of central programs to rapid limb movement in the cat. In: Asanuma H, Wilson VJ (eds) Integration in the nervous system. Igaku-Shoin, Tokyo, pp305–320
Ghez C, Vicario D (1978a) The control of rapid limb movement in the cat. I. Response latency. Exp Brain Res 33: 173–190
Ghez C, Vicario D (1978b) The control of rapid limb movement in the cat. II. Scaling of isometric force adjustments. Exp Brain Res 33: 191–203
Ghez C, Vicario C, Martin JH, Yumiya H (1983) Sensory motor processing of targeted movements in motor cortex. In: Desmedt JE (ed) Motor control in health and disease. Raven Press, New York, pp 61–92
Gottsdanker R (1966) The effect of superseding signals. J Exp Psychol 18: 236–249
Kernell D (1965) The limits of firing frequency in cat lumbosacral motoneurons stimulated by long lasting injected currents. Acta Physiol Scand 65: 65–73
Kuno M (1959) Excitability following antidromic activation in spinal motoneurons supplying red muscles. J Physiol (Lond) 149: 374–393
Megaw ED (1974) Possible modification to a rapid on-going programmed manual response. Brain Res 71: 425–441
Nashner ML (1981) Analysis of stance posture in humans. In: Towe AL, Luschei ES (eds) Handbook of behavioral biology. Plenum Publishing Corp, pp 527–565
Navas F, Stark L (1968) Sampling or intermittency in hand controlled systems dynamics. Biophysic J 8: 252–302
Robinson DA (1970) Oculomotor unit behavior in the monkey. J Neurophysiol 33: 393–404
Robinson DA (1973) Models of the saccadic eye movement control system. Kybernetik 14: 71–83
Soechting JF, Lacquaniti F (1983) Modification of a pointing movement in response to a change in target location. J Neurophysiol 49: 548–564
Telford CW (1931) The refractory phase of voluntary and associative responses. J Exp Psychol 14: 1–36
Thach WT (1978) Correlation of neural discharge with pattern and force of muscular activity, joint position, and direction of the intended movement in motor cortex and cerebellum. J Neurophysiol 41: 654–676
Vicario DS, Martin JH, Ghez C (1983) Specialized subregions in the cat motor cortex: a single unit analysis in the behaving animal. Exp Brain Res 51: 351–367
Vicario DS, Blunk T, Ghez C (1978) Correction of ongoing motor output in the cat. Soc Neurosci Abst 4: 977
Vince MA (1948) The intermittency of control movements and the psychological refractory period. Br J Psychol 38: 149–157
Vince MA (1950) Some exceptions to the psychological refractory period in unskilled manual responses. Med Res Coun Ap Psychol Res U Rep No. 124/50
Vince MA, Welford AT (1967) Time taken to change the speed of a response. Nature 213: 532–533
Welford AT (1959) Evidence of a single-channel decision mechanism limiting performance in a serial reaction task. Q J Exp Psychol 11: 193–210
Welford AT (1967) Single-channel operation in the brain. Acta Psychol 27: 5–22
Welford AT (1980) The single-channel hypothesis. In: Welford AT (ed) Reaction times. Academic Press, London, pp215–252
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vicario, D.S., Ghez, C. The control of rapid limb movement in the cat. Exp Brain Res 55, 134–144 (1984). https://doi.org/10.1007/BF00240507
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00240507