Summary
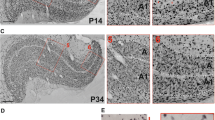
In five, dark-reared, 4-week-old kittens the posterior two thirds of the corpus callosum were split, and a lesion comprising the intralaminar nuclei was made of the left medial thalamic complex. In addition, the right eye was closed by suture. Postoperatively, the kittens showed abnormal orienting responses, neglecting visual stimuli presented in the hemifield contralateral to the side of the lesion. Sudden changes in light, sound, or somatosensory stimulation elicited orienting responses that all tended toward the side of the lesion. These massive symptoms faded within a few weeks but the kittens continued to neglect visual stimuli in the hemifield contralateral to the lesion when a second stimulus was presented simultaneously in the other hemifield. Electrophysiologic analysis of the visual cortex, performed after the end of the critical period, revealed marked interhemispheric differences. In the visual cortex of the normal hemisphere most neurons were monocular and responded exclusively to stimulation of the open eye, but otherwise had normal receptive field properties. In the visual cortex of the hemisphere containing the thalamic lesion, the majority of the neurons remained binocular. In addition, the selectivity for stimulus orientation and the vigor of responses to optimally aligned stimuli were subnormal on this side. Thus, the same retinal signals, which in the control hemisphere suppressed the pathways from the deprived eye and supported the development of normal receptive fields, failed to do either in the hemisphere containing the thalamic lesion. Apparently, experience-dependent changes in the visual cortex require both retinal stimulation and the functioning of diencephalic structures which modulate cortical excitability and control selective attention.
Similar content being viewed by others
References
Akert K, Hartmann von Monakow K (1980) Relationships of precentral, premotor and prefrontal cortex to the mediodorsal and intralaminar nuclei of the monkey thalamus. Acta Neurobiol Exp 40: 7–25
Barlow HB (1975) Visual experience and cortical development. Nature 258: 199–204
Bowker RM, Morrison AR (1976a) The PGO spike: An indicator of hyperalertness. In: Chase MH, Mitler MM, Walter PL (eds) Sleep research, vol 5. UCLA, Los Angeles, pp 23–27
Bowker RM, Morrison AR (1976b) The startle reflex and PGO spikes. Brain Res 102: 185–190
Buisseret P, Gary-Bobo E (1979) Development of visual cortical orientation specificity after dark-rearing: Role of extraocular proprioception. Neurosci Lett 13: 259–263
Buisseret P, Gary-Bobo E, Imbert M (1978) Ocular motility and recovery of orientational properties of visual cortical neurons in dark-reared kittens. Nature 272: 816–817
Crewther SG, Crewther DP, Peck CK, Pettigrew JD (1980) Visual cortical effects of rearing cats with monocular or binocular cyclotorsion. J Neurophysiol 44: 97–118
Cynader M, Mitchell DE (1977) Monocular astigmatism effects on kitten visual cortex development. Nature 270: 177–178
Edwards SB, deOlmos JS (1976) Autoradiographic studies of the projection of the midbrain reticular formation: Ascending projections of nucleus cuneiformis. J Comp Neurol 165: 417–432
Elberger AJ (1979) The role of the corpus callosum in the development of interocular eye alignment and the organization of the visual field in the cat. Exp Brain Res 36: 71–85
Elberger AJ (1981) Ocular dominance in striate cortex is altered by neonatal section of the posterior corpus callosum in the cat. Exp Brain Res 41: 280–291
Freeman RD, Bonds AB (1979) Cortical plasticity in monocularly deprived immobilized kittens depends on eye movement. Science 206: 1093–1095
Fregnac Y, Imbert M (1978) Early development of visual cortical cells in normal and dark-reared kittens: Relationship between orientation selectivity and ocular dominance. J Physiol (Lond) 278: 27–44
Gilbert CD (1977) Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol (Lond) 268: 391–421
Harvey AR (1980) The afferent connexions and laminar distribution in cells of area 18 in the cat. J Physiol (Lond) 302: 483–505
Hebb DO (1949) The organization of behaviour. Wiley & Sons, New York
Hubel DH, Wiesel TN (1962) Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol (Lond) 160: 106–154
Hunsperger RW, Roman D (1976) The integrative role of the intralaminar system of the thalamus in visual orientation and perception in the cat. Exp Brain Res 25: 231–246
Imbert M, Buisseret P (1975) Receptive field characteristics and plastic properties of visual cortical cells in kittens reared with or without visual experience. Exp Brain Res 22: 25–36
Jasper HH, Aimone-Marsan C (1960) A stereotaxic atlas of the diencephalon of the cat. Univ of Toronto Press, Toronto
Jones BE, Moore RY (1977) Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res 127: 23–53
Jones EG (1981) Functional subdivision and synaptic organization of the mammalian thalamus. In: Porter R (ed) Neurophysiology. IV. Int Rev Physiol, vol 25. Univ. Park Press, Baltimore, pp 173–245
Kasamatsu T, Pettigrew JD (1976) Depletion of brain cateholamines: Failure of ocular dominance shift after monocular occlusion in kittens. Science 194: 206–209
Kasamatsu T, Pettigrew JD (1979) Preservation of binocularity after monocular deprivation in the striate cortex of kittens treated with 6-hydroxydopamine. J Comp Neurol 185: 139–162
Kasamatsu T, Pettigrew JD, Ary M (1979) Restoration of visual cortical plasticity by local microperfusion of norepinephrine. J Comp Neurol 185: 163–181
Kennedy H, Baleydier C (1977) Direct projections from thalamic intralaminar nuclei to extrastriate visual cortex in the cat traced with horseradish peroxidase. Exp Brain Res 28: 133–139
Leichnetz GR, Spencer RF, Hardy SGP, Astruc J (1980) The organization of the primate prefrontotectal projection. Exp Brain Res 41: A34
Lindvall O, Bjrklund A (1974) The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand [Suppl] 412: 1–48
McGuinness CM, Krauthamer GM (1978) Afferent projection to the N.centralis lateralis in the cat as demonstrated by retrograde transport of HRP. Neurosci Abstr 4: 555
McGuinness CM, Krauthamer GM (1980) The afferent projections to the centrum medianum of the cat as demonstrated by retrograde transport of horseradish peroxidase. Brain Res 184: 255–269
Miller JW, Benevento LA (1979) Demonstration of a direct projection from the intralaminar central lateral nucleus to the primary visual cortex. Neurosci Lett 14: 229–234
Mitzdorf U, Singer W (1978) Prominent excitatory pathways in the cat visual cortex (A17 and A18): A current source density analysis of electrically evoked potentials. Exp Brain Res 33: 371–394
Mitzdorf U, Singer W (1980) Monocular activation of visual cortex in normal and monocularly deprived cats: An analysis of evoked potentials. J Physiol (Lond) 304: 203–220
Morrison JH, Molliver ME, Coyle JT (1981) The intra-cortical trajectory of the coeruleo-cortical projection in the rat: a tangentially organized cortical afferent. Neuroscience 6: 139–158
Movshon JA, Van Sluyters RC (1981) Visual neural development. Ann Rev Psych 32: 477–522
Orem J, Schlag J (1971) Direct projections from cat frontal eye field to internal medullary lamina of the thalamus. Exp Neurol 33: 509–517
Orem J, Schlag J (1971) Direct projections from cat frontal eye field to internal medullary lamina of the thalamus. Exp Neurol 33: 509–517
Payne BR, Elberger AJ, Berman N, Murphy EH (1980) Binocularity in the cat visual cortex is reduced by sectioning the corpus callosum. Science 207: 1097–1099
Phillis JW (1976) Acetylcholine and synaptic transmission in the central nervous system. In: Hockman CH, Bieger D (eds) Chemical transmission in the mammalian nervous system. Univ. Baltimore Park Press, pp 159–213
Rauschecker JP, Singer W (1979) Changes in the circuitry of the kitten's visual cortex are gated by postsynaptic activity. Nature 280: 58–60
Rauschecker JP, Singer W (1981) The effects of early visual experience on the cat's visual cortex and their possible explanation by Hebb synapses. J Physiol (Lond) 310: 215–239
Reinoso-Suarez F (1961) Topographischer Hirnatlas der Katze. E. Merck, Darmstadt
Ropert N, Steriade M (1981) Input-output organization of midbrain reticular core. J Neurophysiol 46: 17–31
Scheibel ME, Scheibel AB (1958) Structural substrates for integrative patterns in brain stem reticular core. In: Jasper HH (ed) Reticular formation of the brain. Little, Brown & Co., Boston, pp 31–55
Schlag J, Schlag-Rey M (1971) Induction of oculomotor responses from thalamic internal medullary lamina in the cat. Exp Neurol 33: 498–508
Schlag J, Schlag-Rey M (1980) Receptive field location, direction of gaze and stimulus absolute position as determinants of visual responses in thalamic gaze neurons. Exp Brain Res 41: A35
Schlag J, Lethinen J, Schlag-Rey M (1974) Neuronal activity before and during eye movement in thalamic medullary lamina of the cat. J Neurophysiol 37: 982–995
Schlag J, Schlag-Rey M, Peck CK, Joseph JP (1980) Visual responses of thalamic neurons depending on the direction of gaze and the position of targets in space. Exp Brain Res 40: 170–184
Schlag-Rey M, Schlag J (1977) Visual and presaccadic neuronal activity in thalamic internal medullary lamina of cat: A study of targeting. J Neurophysiol 40: 156–173
Shatz CJ, Lindstrom S, Wiesel TN (1977) The distribution of afferents representing the right and left eyes in the cats visual cortex. Brain Res 131: 103–116
Singer W (1978) The effect of monocular deprivation on cat parastriate cortex: Asymmetry between crossed and uncrossed pathways. Brain Res 157: 351–355
Singer W (1979) Central-core control of visual cortex functions. In: Schmitt FO, Worden FG (eds) The neurosciences, fourth study program. MIT Press, Cambridge, MA, pp 1093–1109
Singer W, Rauschecker J (1982) Central core control of developmental plasticity in the kitten visual cortex: II. Electrical activation of mesencephalic and diencephalic projections. Exp Brain Res 47: 223–233
Singer W, Tretter F, Cynader M (1975) Organization of cat striate cortex: A correlation of receptive field properties with afferent and efferent connections. J Neurophysiol 38: 1080–1098
Singer W, Tretter F, Cynader M (1976) The effect of reticular stimulation on spontaneous and evoked activity in the cat visual cortex. Brain Res 102: 71–90
Singer W, Rauschecker J, Werth R (1977) The effect of monocular exposure to temporal contrasts on ocular dominance in kittens. Brain Res 134: 568–572
Singer W, Yinon U, Tretter F (1979a) Inverted monocular vision prevents ocular dominance shift in kittens and impairs the functional state of visual cortex in adult cats. Brain Res 164: 294–299
Singer W, von Grunau MW, Rauschecker J (1979b) Requirements for the disruption of binocularity in the visual cortex of strabismic kittens. Brain Res 171: 536–540
Singer W, von Grunau MW, Rauschecker J (1980) Functional amblyopia in kittens with unilateral exotropia: I. Electrophysiological assessment. Exp Brain Res 40: 294–304
Singer W, Tretter F, Yinon U (1982) Central gating of developmental plasticity in kitten visual cortex. J Physiol 324: 221–237
Steriade M (1981) Mechanisms underlying cortical activation: Neuronal organization and properties of the midbrain reticular core and intralaminar thalamic nuclei. In: Pompeiano O, Ajmone Marsan C (eds) Brain mechanisms and perceptual awareness. Raven Press, New York, pp 327–377
Tretter F, Cynader M, Singer W (1975) Cat parastriate cortex: A primary or secondary visual area? J Neurophysiol 38: 1098–1113
Veazey RB, Severin CM (1980) Efferent projections of the deep mesencephalic nucleus (pars lateralis) in the rat. J Comp Neurol 190: 231–244
Watson RT, Heilman KM (1979) Thalamic neglect. Neurology 29: 690–694
Wurtz RH, Albano JE (1980) Visual-motor function of the primate superior colliculus. Ann Rev Neurosci 3: 189–226
Wurtz RH, Goldberg ME, Robinson DL (1980) Behavioral modulation of visual response in the monkey: Stimulus selection for attention and movement. Prog Psychobiol Physiol Psychol 9: 43–83
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Singer, W. Central core control of developmental plasticity in the kitten visual cortex: I. Diencephalic lesions. Exp Brain Res 47, 209–222 (1982). https://doi.org/10.1007/BF00239380
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00239380