Abstract
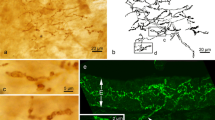
To study the configuration of myoepithelial cells, we isolated glandular endpieces of various guinea pig glands by collagenase, and visualized the myoepithelial cells by immunohistochemistry for actin, or by Bodipy-phallacidin, under both a light microscope and laser scanning confocal microscopes. In parotid and mandibular glands, the glandular acini were small (about 20–30 μm diameter) and spherical, and each acinus had one or two myoepithelial cells attached that were stellate in shape (central cell body and four to six thin processes). Most of the basal surface of the glandular cells was not covered by myoepithelial cells, and processes often extended to the neighboring acinus. The tubular glandular endpieces of the major sublingual gland, which secretes a mucous substance, were almost fully encircled by bandlike myoepithelial cells (about 3–6 μm wide). Although there were many differences between the lacrimal gland and the Harderian gland (e.g., the secretory product of the lacrimal gland was mucous, and glandular lumina were narrow; the Harderian gland secreted lipids and showed wide lumina), the outer contours of both glandular endpieces were the same (about 50–100 μm diameter, ellipsoid or spherical in shape). In both glands, 5–20 stellate myoepithelial cells were attached onto a glandular endpiece, and their arrangement had a lacy appearance. Actin filaments in myoepithelial cells aggregated and formed bundles in the broad processes and cell bodies. The bundles ran across the cell body, but there was no point where the bundles converged. In the arborization, some distal processes reversed their direction. We conclude that the configuration of myoepithelial cells depends on the outer contour of the glandular endpieces rather than on the secretory material or luminal width. The variety of myoepithelial cell configurations in the different exocrine glands we examined suggests that it is quite difficult to assign to myoepithelial cells the general role of expelling secretory products from glandular lumina. These cells seem to maintain the contour of the glandular endpieces, serving as the exoskeleton of the endpieces.
Similar content being viewed by others
References
Abe J, Sugita A, Katsume Y, Yoshizuka M, Tamura N, Iwanaga S, Nishida T (1980) Scanning electron microscopic observations of the Harderian gland in rat. Kurume Med J 27:239–246
Abe J, Sugita A, Hamasaki M, Nakamura K, Iwanaga S, Nagae K, Atsuji K, Tsunawaki A, Abe T, Matsumoto T, Yo S, Murakami M (1981) Scanning electron microscopic observations of the myoepithelial cells of normal and contracting status in the rat Harderian gland. Kurume Med J 28:103–112
Archer FL, Kao VCY (1968) Immunohistochemical identification of actomyosin in myoepithelium of human tissues. Lab Invest 18:669–674
Brocco SL, Tamarin A (1979) The topography of rat submandibular gland parenchyma as observed with SEM. Anat Rec 194:445–460
Ellis R (1965) Fine structure of the myoepithelium of the eccrine sweat glands of man. J Cell Biol 27:551–563
Emerman JT, Vogl W (1986) Cell size and shape changes in the myoepithelium of the mammary gland during differentiation. Anat Rec 216:405–415
Fawcett DW (1986) A text book of histology, 11th edn. Saunders, Philadelphia London Toronto
Furuya S, Furuya K (1993) Characteristics of cultured subepithelial fibroblasts of rat duodenal villi. Anat Embryol 187:529–538
Garrett JR, Emmelin N (1979) Activities of salivary myoepithelial cells. Med Biol 57:1–28
Garrett JR, Harrison JD (1970) Alkaline-phosphatase and adenosine-triphosphatase histochemical reactions in the salivary glands of cat, dog, and man, with particular reference to the myoepithelial cells. Histochemie 24:214–229
Habara Y, Satoh Y, Saito T, Kanno T (1990) A G-protein activator, NaF, induces [Ca2+]0-dependent [Ca2+] oscillation and secretory response in rat pancreatic acini. Biomed Res 11:389–398
Hootman SR, Brown ME, Williams JA, Logsdon CD (1986) Regulation of muscarinic acetylcholine receptors in cultured guinea pig pancreatic acini. Am J Physiol 251:G75-G83
Hsu S-M, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedure. J Histochem Cytochem 29:577–580
Kawabata I, Kurosumi K (1976) Transmission and scanning electron microscopy of the human ceruminous apocrine gland II. Myoepithelial cells. Arch Histol Jpn 39:231–255
Krstić RV (1984) Illustrated encyclopedia of human histology. Springer, Berlin Heidelberg New York
Kurosumi K, Shibasaki S, Ito T (1984) Cytology of the secretion in mammalian sweat glands. Int Rev Cytol 87:253–329
Leeson CR (1960) The histochemical identification of myoepithelium, with particular reference to the Harderian and exorbital lacrimal glands. Acta Anat 40:87–94
Leeson TS, Leeson CR (1971) Myoepithelial cells in the exorbital lacrimal and parotid glands of the rat in frozen-etched replicas. Am J Anat 132:133–146
López JM, Alvarez-Uría M (1993) A morphometric and fine structural study of the myoepithelial cells in the hamster Harderian gland. J Submicrosc Cytol Pathol 25:223–232
Lôpez JM, Tolivia J, Alvarez-Uría M (1992) An ultrastructural study of myoepithelium maturation during postnatal development of the hamster Harderian gland. Anat Embryol 186:573–582
Moore DM, Vogl AW, Baimbridge K, Emerman JT (1987) Effect of calcium on oxytocin-induced contraction of mammary gland myoepithelium as visualized by NBD-phallacidin. J Cell Sci 88:563–569
Murakami M, Sugita A, Abe J, Hamasaki M, Shimada T (1981) SEM observation of some exocrine glands, with special reference to configuration of the associated myoepithelial cells. Biomed Res 2 [Suppl]:99–102
Nagashima Y, Ono K (1985) Myoepithelial cell ultrastructure in the submandibular gland of man. Anat Embryol 171:259–265
Nagato T, Yoshida H, Yoshida A, Uehara Y (1980) A scanning electron microscope study of myoepithelial cells in exocrine glands. Cell Tissue Res 209:1–10
Nomina Histologica (1983) In: Nomina Anatomica, 5th edn. Williams & Wilkins, Baltimore
Norberg L, Dardick I, Leung R, Burford-Mason AP, Rippstein P (1992) Immunogold localization of actin and cytokeratin filaments in myoepithelium of human parotid salivary gland. Ultrastruct Pathol 16:555–568
Pitelka DR, Hamamoto ST, Duafala JG, Nemanic MK (1973) Cell contacts in the mouse mammary gland. I. Normal gland in postnatal development and the secretory cycle. J Cell Biol 56:797–818
Radner CJP (1972) Myoepithelial cell differentiation in rat mammary glands. J Anat 111:381–398
Redman RS, Sweney LR, McLaughlin ST (1980) Differentiation of myoepithelial cells in the developing rat parotid gland. Am J Anat 158:299–320
Satoh Y, Saino T, Ono K (1990) Effect of carbamylcholine on Harderian gland morphology in rats. Cell Tissue Res 261:451–459
Satoh Y, Habara Y, Kanno T, Ono K (1993) Carbamylcholineinduced morphological changes and spatial dynamics of [Ca2+] in Harderian glands of guinea pigs: calcium-dependent lipid secretion and contraction of myoepithelial cells. Cell Tissue Res 274:1–14
Tamarin A (1966) Myoepithelium of the rat submaxillary gland. J Ultrastruct Res 16:320–338
Tashiro S, Smith CC, Badger E, Kezur E (1940) Chromodacryorrhea, a new criterion for biological assay of acetylcholine. Proc Soc Exp Biol 44:658–661
Verdugo P (1990) Goblet cells secretion and mucogenesis. Ann Rev Physiol 52:157–176
Weiss L (1983) Histology, 5th edn. Elsevier, New York
Wulf E, Deboben A, Bautz FA, Faulstich H, Wieland T (1979) Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci USA 76:4498–4502
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Satoh, Y., Oomori, Y., Ishikawa, K. et al. Configuration of myoepithelial cells in various exocrine glands of guinea pigs. Anat Embryol 189, 227–236 (1994). https://doi.org/10.1007/BF00239010
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00239010