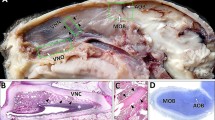
Summary
The sensory neurons of the olfactory epithelium, as a consequence of their odor detection function, contact both the external environment and the central nervous system. The possibility that substances applied to the epithelium might reach the central nervous system was investigated by the intranasal application of peroxidase-conjugated wheat germ agglutinin (WGA-HRP). WGA-HRP was transported through olfactory receptor axons to the glomerulus of the olfactory bulb. Reaction product was localized electron microscopically to tubulovesicular profiles and dense bodies in sensory axons. Evidence of transneuronal transport was indicated by reaction product localized in dense bodies in dendrites postsynaptic to receptor cell axons. Periglomerular, tufted and mitral cells in the olfactory bulb also were transneuronally labeled. Anterograde transneuronal labeling occured in the olfactory tubercle, piriform cortex and surrounding the lateral olfactory tract. Retrograde transneuronal label was found in neurons of the basal forebrain with the largest number of perikarya in the lateral nucleus of the horizontal limb of the diagonal band, a major source of cholinergic afferents to the olfactory bulb. These data suggest that substances, specifically those which bind to receptors, are transported from the olfactory receptor neurons in the nasal epithelium to the brain. Thus, the olfactory system may provide a route of entry for exogenous substances to the basal forebrain.
Similar content being viewed by others
Abbreviations
- AC:
-
anterior commissure
- CC:
-
corpus callosum
- CI:
-
internal capsule
- CP:
-
caudate putamen
- DBB:
-
diagonal band of Broca
- FX:
-
fornix
- GP:
-
globus pallidus
- IC:
-
island of Callelae
- LV:
-
lateral ventricle
- MS:
-
medial septum
- OC:
-
optic chiasm
- PIR:
-
piriform cortex
- RF:
-
rhinal fissure
- SON:
-
supraoptic nucleus
- SCN:
-
suprachiasmatic nucleus
- SM:
-
stria medullaris
- ST:
-
stria terminalis
- TOL:
-
lateral olfactory tract
- TUO:
-
olfactory tubercle
- III:
-
third ventricle
References
Adamek GD, Gesteland RC, Mair RG, Oakley B (1984) Transduction physiology of olfactory receptor cilia. Brain Res 310: 87–97
Allen WK, Akeson R (1985) Identification of a cell surface glycoprotein family of olfactory receptor neurons with a monoclonal antibody. J Neurosci 5: 284–296
Anholt RRH, Murphy KMM, Mack GE, Snyder SH (1984) Peripheral-type benzodiazepine receptors in the central nervous system: localization to olfactory nerves. J Neurosci 4: 593–603
Baker H, Kawano T, Margolis FL, Joh TH (1983) Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci 3: 69–78
Baker H, Kawano T, Joh TH, Margolis FL, Reis DJ (1984) Tyrosine hydroxylase deficient dopamine neurons survive peripheral deafferentation of the olfactory bulb. Neuroscience 11: 605–615
Bartus RT, Dean RL III, Beer B, Zippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408–417
Bondareff W, Mountjoy CQ, Roth M (1981) Selective loss of neurons of origin of adrenergic projection to cerebral cortex (nucleus locus coeruleus) in senile dementia. Lancet i 783–784
Bowen DM, Allen, SJ, Benton JS, Goodhardt MJ, Haan EA, Palmer AM, Sims NR, Smith CCT, Spillane JA, Esiri MM, Neary D, Snowdon JS, Wilcock GK, Davison AN (1983) Biochemical assessment of serotonergic and cholinergic dysfunction and cerebral atrophy in Alzheimer's disease. J Neurochem 41: 266–272
Broadwell RD (1975) Olfactory relationships of the telencephalon and diencephalon in the rabbit. I. An autoradiographic study of the efferent connections of the main and accessory olfactory bulbs. J Comp Neurol 163: 329–346
Broadwell RD, Jacobowitz DM (1976) Olfactory relationships of the telencephalon and diencephalon in the rabbit. J Comp Neurol 170: 321–346
Broadwell RD, Cataldo AM, Balin BJ (1984) Further studies of the secretory process in hypothalamus-neurohypophyseal neurons: an analysis using immunocytochemistry, wheat germ agglutinin-peroxidase, and native peroxidase. J Comp Neurol 228: 155–167
Carson KA, Burd GD (1980) Localization of acetylcholinesterase in the main and accessory olfactory bulbs of mouse by lightand electron-microscopic histochemistry. J Comp Neurol 191: 353–371
Chuah MI, Farbman AI (1983) Olfactory bulb increases marker protein in olfactory receptor cells. J Neurosci 3: 2197–2205
Corwin J, Serby M, Conrad P, Rotrosen J (1985) Olfactory recognition deficit in Alzheimer's and Parkinsonian dementias. IRCS Med Sci 13: 260
Coyle JT, Price DL, DeLong MR (1983) Alzheimer's disease: a disorder of cortical cholinergic innervation. Science 219: 1184–1190
de Lorenzo AJD (1970) The olfactory neuron and the blood brain barrier. In: Wolstenholme GEW, Knight J (eds) Taste and smell in vertebrates. Churchill, London, pp 151–175
de Olmos J, Hardy H, Heimer L (1978) The afferent connections of the main and the accessory olfactory bulb formation in the rat. An experimental HRP study. J Comp Neurol 181: 213–244
Davis BJ, Macrides F (1981) The organization of centrifugal projections from the anterior olfactory nucleus, ventral hippocampal rudiment and piriform cortex to the main olfactory bulb in the hamster: an autoradiographic study. J Comp Neurol 203: 475–493
Doucette JR (1984) The glial cells in the nerve fiber layer of the rat olfactory bulb. Anat Rec 210: 385–391
Eseri MM, Tomlinson AH (1984) Herpes simplex encephalitis. J Neurol Sci 64: 213–217
Eseri MM, Wilcock GK (1984) The olfactory bulbs in Alzheimer's disease. J Neurol Neurosurg Psychiat 47: 56–60
Farbman AI, Margolis FL (1980) Olfactory marker protein during ontogeny: immunohistochemical localization. Dev Biol 74: 207–215
Fibiger HC (1982) The organization and some projections of cholinergic neurons of the mammalian forebrain. Brain Res Rev 4: 327–388
Gottfries CG (1982) The metabolism of some neurotransmitters in ageing and dementia disorders. Gerontology 28: 11–19
Graziadei PPC, Monti-Graziadei GA (1978) The olfactory system: a model for the study of neurogenesis and axon regeneration in mammals. In: Cotman CC (ed) Neuronal plasticity, Raven Press, New York, pp 131–153
Hedlund B, Shepherd GM (1983) Biochemical studies on muscarinic receptors in the salamander olfactory epithelium. FEBS 162: 428–431
Hinds JW, McNelly NA (1981) Aging in the rat olfactory system: correlation of changes in the olfactory epithelium and olfactory bulb. J Comp Neurol 203: 441–453
Hirsch JD, Margolis FL (1981) Isolation, separation and analysis of cells from olfactory epithelium. In: Cagan RH, Kare MR (eds) Biochemistry of taste and olfaction. Academic Press, New York, pp 331–332
Holl A (1980) Selective staining by Procion dyes of olfactory sensory neurons in the catfish Ictalurus nebulosus Z. Naturforsch 35: 526–528
Hornykiewicz O (1983) Parkinson's disease: from brain homogenate to treatment. Fed Proc 32: 183–190
Itaya SK, Van Hoesen GW (1982) WGA HRP as a transneuronal marker in the visual pathways of monkey and rat. Brain Res 236: 199–204
Itoh K, Konishi A, Nomura S, Mizuno N, Nakamura Y, Sujimoto T (1979) Application of coupled oxidation reaction to electron microscopic demonstration of horseradish peroxidase: cobaltglucose oxidase method. Brain Res 175: 341–346
Itoh K, Yasui Y, Takada M, Mitani A, Kaneko T, Sugimoto T, Mizuno N (1984) An anterograde-retrograde transneuronal transport of conjugates of wheat germ agglutinin with horseradish peroxidase (WGA-HRP): labeling of neurons in the reticular nucleus of the thalamus with WGA-HRP injected into the posterior column nuclei in the cat. Brain Res 323: 185–187
Iversen LL, Rossor MN, Reynolds GP, Hills R, Roth M, Mountjoy CQ, Foote SL, Morrison JH, Bloom FE (1983) Loss of pigmented dopamine β-hydroxylase positive cells from locus coeruleus in senile dementia of Alzheimer's type. Neurosci Lett 39: 95–100
Jackson RT, Tigges J, Arnold W (1979) Subarachnoid space of the CNS, nasal mucosa, and lymphatic system. Arch Otolaryngol 105: 180–184
Kimura H, McGeer PL, Peng JH, McGeer EG (1981) The central cholinergic system studied by cholineacetyltransferase immunohistochemistry in the cat. J Comp Neurol 200: 151–201
Kream RM, Davis BJ, Kawano T, Margolis FL, Macrides F (1984) Substance P and catecholaminergic expression in neurons of the hamster main olfactory bulb. J Comp Neurol 222:140–154
Kristensson K, Olsson Y (1971) Uptake of exogenous proteins in mouse olfactory cells. Acta Neuropath (Berl) 19: 145–154
Macrides F, Davis BJ, Youngs WM, Nadi NS, Margolis FL (1981) Cholinergic and catecholaminergic afferents to the olfactory bulb in the hamster: a neuroanatomical, biochemical, and histochemical investigation. J Comp Neurol 203: 495–514
Margolis FL (1972) A brain protein unique to the olfactory bulb. Proc Natl Acad Sci USA 69: 1221–1224
McGeer PL, McGeer EG, Suzuki J, Dolman CE, Nagai T (1984) Aging, Alzheimer's disease, and the cholinergic system of the basal forebrain. Neurol 34: 741–745
Mesulam M-M (1978) Tetramethylbenzidine for horseradish peroxidase neurohistochemistry: A non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26: 106–117
Mesulam M-M, Mufson EJ, Wainer BH, Levey AI (1983) Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 10: 1185–1201
Monath TP, Cropp CB, Harrison AK (1983) Mode of entry of a neurotropic arbovirus into the central nervous system. Lab Invest 48: 399–410
Peschanski M, Ralston HJ III (1985) Light and electron microscopic evidence of transneuronal labeling with WGA-HRP to trace somatosensory pathways to the thalamus. J Comp Neurol 236: 29–41
Rhein LD, Cagan RH (1981) Role of cilia in olfactory recognition. In: Cagan RH, Kare MR (eds) Biochemistry of taste and olfaction. Academic Press, New York, pp 47–68
Rossor MN, Svendsen C, Hunt SP, Mountjoy CQ, Roth M, Iversen LL (1982) The substantia innominata in Alzheimer's disease: an histochemical and biochemical study of cholinergic marker enzymes. Neurosci Lett 28: 217–222
Ruda M, Coulter JD (1982) Axonal and transneuronal transport of wheat germ agglutinin demonstrate by immunocytochemistry. Brain Res 249: 237–246
Saper CB (1984) Organization of cerebral cortical afferent systems in the rat. I magnocellular basal nucleus. J Comp Neurol 222: 313–342
Shipley MT, Adamek GD (1984) The connections of the mouse olfactory bulb: a study using orthograde and retrograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. Brain Res Bull 12: 669–688
Spencer RF, Baker H, Baker R (1982) Evaluation of wheat germ agglutinin immunohistochemistry as a neuroanatomical method for retrograde, anterograde and anterograde transsynaptic labeling in the cat visual occulomotor systems. Soc Neurosci Abst 8: 785
Stevens JC, Bartoshuk LM, Cain WS (1984) Chemical senses and aging: taste versus smell. Chemical Senses 9: 167–179
Stroop WG, Rock DL, Fraser NW (1984) Localization of herpes simplex virus in the trigeminal and olfactory systems of the mouse central nervous system during acute and latent infections by in situ hybridization. Lab Invest 51: 27–38
Tomlinson BE, Irving D, Blessed G (1981) Cell loss in the locus coeruleus in senile dementia of Alzheimer type. J Neurol Sci 49: 419–428
Trojanowski JQ (1983) Native and derivatized lectins for in vivo studies of neuronal connectivity and neuronal cell biology. J Neurosci Meth 9: 185–204
Weiss P, Holland Y (1967) Neuronal dynamics and axonal flow in the olfactory nerve as model test object. Proc Natl Acad Sci (USA) 57: 258–264
Yates CM, Simpson J, Gordon A, Maloney AFJ, Allison Y, Ritchie IM, Urquhart A (1983) Catecholamines and cholinergic enzymes in pre-senile and senile Alzheimer-type dementia and Down's syndrome. Brain Res 280: 119–126
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Baker, H., Spencer, R.F. Transneuronal transport of peroxidase-conjugated wheat germ agglutinin (WGA-HRP) from the olfactory epithelium to the brain of the adult rat. Exp Brain Res 63, 461–473 (1986). https://doi.org/10.1007/BF00237470
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00237470