Summary
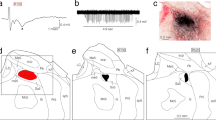
Extracellular and intracellular recordings were made from spinocerebellar tract neurones of the central cervical nucleus (CCN) in C1–C3 segments of the anaesthetized cat. These neurones were identified by antidromic activation from the cerebellar peduncle. Stimulation of the ipsilateral dorsal root elicited extracellular spikes or EPSPs with a monosynaptic latency in almost all CCN neurones in the same segment (segmental input). Late excitatory effects were also observed in about one third of CCN neurones. The monosynaptic EPSP was occasionally followed by an IPSP. The excitatory input from the dorsal root to CCN neurones was extended over several segments for some CCN neurons (extrasegmental input). Monosynaptic excitation was evoked in CCN neurones after stimulation of dorsal neck muscle nerves as well; i.e. splenius (SPL), biventer cervicis and complexus (BCC), rectus capitus dorsalis, and obliquus capitus caudalis. Thresholds for this excitation were near the threshold of the nerve, suggesting that it originated from group I fibres. The component of excitation added after strong stimulation of neck muscle nerves would be attributed to group II fibres. When a CCN neurone received excitatory input from the nerve of one muscle, it was generally not affected by stimulation of other nerves in the same segment. Such muscle specificity of segmental input was the principal pattern of connexion of neck muscle afferents with CCN neurones. In some cases, however, excitatory convergence from SPL and BCC nerves onto single CCN neurones or excitation from the SPL nerve and inhibition from the BCC nerve were also observed. Nearly half of the CCN neurones received input from one muscle nerve of the same segment and not from the afferent of the same muscle of different segments, indicating a segment specificity of input. In the remaining CCN neurones, weaker excitatory effects were induced from afferents of different segments as well. In such extrasegmental effects, inputs to CCN neurones from caudal segments predominated in frequency over those from rostral segments. The origin of extrasegmental input was generally confined to the same muscle. Low threshold muscle afferents from the SPL and BCC were intraaxonally stained with HRP. The collaterals of the stained fibre distributed branchlets and terminals to the CCN, laminae VII, VIII, and motor nuclei. Two fibres responding to local muscle prodding or stretch showed a similar morphology. The findings indicated that muscle spindle afferents from primary endings projected to the CCN.
Similar content being viewed by others
References
Abrahams VC, Anstee G, Richmond FJR, Rose PK (1979) Neck muscle and trigeminal input to the upper cervical cord and lower medulla of the cat. Can J Physiol Pharmacol 57: 642–651
Anderson ME (1977) Segmental reflex inputs to motoneurons innervating dorsal neck musculature in the cat. Exp Brain Res 28: 175–187
Brink EE, Jinnai K, Wilson VJ (1981) Pattern of segmental monosynaptic input to cat dorsal neck motoneurons. J Neurophysiol 46: 496–505
Brown AG (1981) Organization in the spinal cord. The anatomy and physiology of identified neurones. Springer, Berlin
Corbin, KB, Hinsey JC (1935) Intramedullar course of the dorsal root fibers of each of the first four cervical nerves. J Comp Neurol 63: 119–126
Cummings, JF, Petras JM (1977) The origin of spinocerebellar pathways. 1. The nucleus cervicalis centralis of the cranial cervical spinal cord. J Comp Neurol 173: 655–692
Eccles, JC, Oscarsson O, Willis WD (1961) Synaptic action of group I and II afferent fibres of muscle on the cells of the dorsal spinocerebellar tract. J Physiol (Lond) 158: 517–543
Escolar J (1948) The afferent connections of the 1st, 2nd, 3rd cervical nerves in the cat. J Comp Neurol 89: 79–92
Ezure K, Sasaki S, Uchino Y, Wilson VJ (1978) Frequency-response analysis of vestibular-induced neck reflex in cat. II. Functional significance of cervical afferents and polysynaptic descending pathways. J Neurophysiol 41: 459–471
Fyffe REW (1979) The morphology of group II muscle afferent fibre collaterals. J Physiol (Lond) 296: 39P-40P
Gustafsson B, Lindström S (1973) Recurrent control from motor axon collaterals of Ia inhibitory pathways to ventral spinocerebellar tract neurones. Acta Physiol Scand 89: 457–481
Hirai N, Hongo T, Sasaki S (1978) Cerebellar projection and input organization of the spinocerebellar tract arising from the central cervical nucleus in the cat. Brain Res 157: 341–345
Hirai N, Hongo T, Sasaki S, Yoshida K (1979) The neck and labyrinthine influences on cervical spinocerebellar tract neurones of the central cervical nucleus in the cat. Prog Brain Res 50: 529–536
Hirai N, Hongo T, Sasaki S (1984) A physiological study of identification, axonal course and cerebellar projection of spinocerebellar tract cells in the central cervical nucleus in the cat. Exp Brain Res 55: 272–285
Holmqvist B, Lundberg A, Oscarsson O (1956) Functional organization of the dorsal spinocerebellar tract in the cat. V. Further experiments on convergence of excitatory and inhibitory actions. Acta Physiol Scand 38: 76–90
Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K (1983a) Inhibition of dorsal spinocerebellar tract cells by interneurones in upper and lower lumbar segments in the cat. J Physiol (Lond) 342: 145–159
Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K (1983b) The same interneurones mediate inhibition of dorsal spinocerebellar tract cells and lumbar motoneurones in the cat. J Physiol (Lond) 342: 161–180
Hongo T, Okada Y (1967) Cortically evoked pre- and postsynaptic inhibition of impulse transmission to the dorsal spinocerebellar tract. Exp Brain Res 3: 163–177
Imai Y, Kusama T (1969) Distribution of the dorsal root fibers in the cat. An experimental study with the Nauta method. Brain Res 13: 338–359
Ishizuka N, Mannen H, Hongo T, Sasaki S (1979) Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: Three dimensional reconstructions from serial sections. J Comp Neurol 186: 189–211
Kirkwood PA, Sears TA (1975) Monosynaptic excitation of motoneurones from muscle spindle secondary endings of intercostal and triceps surae muscles in the cat. J Physiol (Lond) 245: 64P-66P
Kuno M, Munoz-Martinez EJ, Randic M (1973) Sensory inputs to neurones in Clarke's column from muscle, cutaneous and joint receptors. J Physiol (Lond) 228: 327–342
Lindström S, Schomburg ED (1973) Group I inhibition in Ib excited ventral spinocerebellar tract neurones. Acta Physiol Scand 90: 166–185
Lundberg A, Weight F (1981) Functional organization of connexions to the ventral spinocerebellar tract. Exp Brain Res 12: 295–316
Lundberg A (1971) Function of ventral spinocerebellar tract. A new Hypothesis. Exp Brain Res 12: 317–330
Mannen H, Ishizuka N, Hongo T, Kudo N, Sasaki S, Yamashita M (1981) Quantitative analysis of the morphology of group II fibers in the spinal cord of the cat. Neurosci Lett Suppl 6: S96
Matsushita M, Ikeda M (1975) The central cervical nucleus as cell origin of a spinocerebellar tract arising from the cervical cord: a study in the cat using horseradish peroxidase. Brain Res 100: 412–417
Matsushita M, Hosoya Y, Ikeda M (1979) Anatomical organization of the spinocerebellar system in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol 184: 81–106
Petras JM (1965) Afferent peripheral nerve fibers to the spinal cord and dorsal column nuclei in the cat. An analysis and comparison with the distribution of terminal efferent brain fibers to the spinal cord. Anat Record 151: 399–400
Petras JM (1977) Spinocerebellar neurons in the rhesus monkey. Brain Res 130: 146–151
Ranson SW, Davenport HK, Doles EA (1932) Intramedullary course of the dorsal root fibers of the first three cervical nerves. J Comp Neurol 54: 1–12
Rapoport S (1979) Reflex connexions of motoneurones of muscles involved in head movement in the cat. J Physiol (Lond) 289: 311–327
Reighard J, Jennings HS (1935) In: Elliott R (ed) Anatomy of the Cat. Holt, Rinehart and Winston, New York, pp 131–136
Rexed B (1954) A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol 100: 297–379
Richmond FJR, Abrahams VC (1979) Physiological properties of muscle spindles in dorsal neck muscles of the cat. J Neurophysiol 42: 604–617
Richmond FJR, Bakker DA (1982) Anatomical organization and sensory receptor content of soft tissues surrounding upper cervical vertebrae in the cat. J Neurophysiol 48: 49–61
Shriver JE, Stein BM, Carpenter MB (1968) Central projections of spinal dorsal roots in the monkey. I. Cervical and upper thoracic dorsal roots. Am J Anat 123: 27–74
Stauffer EK, Watt DGD, Taylor A, Reinking RM, Stuart DG (1976) Analysis of muscle receptor connections by spike triggered averaging. 2. Spindle group II afferents. J Neurophysiol 39: 1393–1402
Wiksten B (1975) The central cervical nucleus — a source of spinocerebellar fibres, demonstrated by retrograde transport of horseradish peroxidase. Neurosci Lett 1: 81–84
Wiksten B (1979a) The central cervical nucleus in the cat. I. A Golgi study. Exp Brain Res 36: 143–154
Wiksten B (1979b) The central cervical nucleus in the cat. II. The cerebellar connections studied with retrograde transport of horseradish peroxidase. Exp Brain Res 36: 155–173
Wilson VJ, Maeda M (1974) Connections between semicircular canals and neck motoneurons in the cat. J Neurophysiol 37: 346–357
Author information
Authors and Affiliations
Additional information
Supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan
Rights and permissions
About this article
Cite this article
Hirai, N., Hongo, T., Sasaki, S. et al. Neck muscle afferent input to spinocerebellar tract cells of the central cervical nucleus in the cat. Exp Brain Res 55, 286–300 (1984). https://doi.org/10.1007/BF00237279
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00237279