Abstract
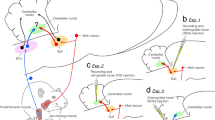
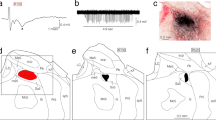
The oval paracentral nucleus (OPC) was initially isolated from the paracentral nucleus (PC) within the intralaminar thalamic nuclei in rats. We have recently shown that the rat OPC receives proprioceptive inputs from jaw-closing muscle spindles (JCMSs). However, it remains unknown which cortical areas receive thalamic inputs from the OPC, and whether the cortical areas receiving the OPC inputs are distinct from those receiving inputs from the other intralaminar nuclei and sensory thalamic nuclei. To address this issue, we injected an anterograde tracer, biotinylated dextranamine (BDA), into the OPC, which was electrophysiologically identified by recording of proprioceptive inputs from the JCMSs. Many BDA-labeled axonal fibers and terminals from the OPC were ipsilaterally observed in the rostral and rostroventral regions of the primary somatosensory cortex (S1), the rostral region of the secondary somatosensory cortex (S2), and the most rostrocaudal levels of the granular insular cortex (GI). In contrast, a BDA injection into the caudal PC, which was located slightly rostral to the OPC, resulted in ipsilateral labeling of axonal fibers and terminals in the rostrolateral region of the medial agranular cortex and the rostromedial region of the lateral agranular cortex. Furthermore, injections of a retrograde tracer, Fluorogold, into these S1, S2, and GI regions, resulted in preferential labeling of neurons in the ipsilateral OPC among the intralaminar and sensory thalamic nuclei. These findings reveal that the rat OPC has widespread, but strong corticopetal projections, indicating that there exist divergent corticopetal pathways from the intralaminar thalamic nucleus, which process JCMS proprioceptive sensation.









Similar content being viewed by others
Abbreviations
- 5C:
-
Trigeminal sensory nucleus caudalis
- 5I:
-
Trigeminal sensory nucleus interpolaris
- 12:
-
Hypoglossal nucleus
- ABC:
-
Avidin–biotin-peroxidase complex
- ac:
-
Anterior commissure
- Acb:
-
Accumbens nucleus
- AcbC:
-
Core part of the Acb
- AcbS:
-
Shell part of the Acb
- ACg:
-
Anterior cingulate cortex
- Agl:
-
Lateral agranular cortex
- Agm:
-
Medial agranular cortex
- AI:
-
Agranular insular cortex
- BDA:
-
Biotinylated dextranamine
- cc:
-
Corpus callosum
- CeA:
-
Central amygdaloid nucleus
- Cl:
-
Claustrum
- CL:
-
Centrolateral thalamic nucleus
- CM:
-
Central medial thalamic nucleus
- CPu:
-
Caudate putamen
- Cu:
-
Cuneate nucleus
- DAB:
-
Diaminobenzidine
- dGIrvs2:
-
Dorsal part of GI rostroventrally adjacent to the rostralmost part of S2
- DI:
-
Dysgranular insular cortex
- DLO:
-
Dorsolateral orbital cortex
- DP:
-
Dorsal peduncular cortex
- ECu:
-
External cuneate nucleus
- FG:
-
Fluorogold
- fr:
-
Fasciculus retroflexus
- GI:
-
Granular insular cortex
- ic:
-
Internal capsule
- IL:
-
Infralimbic cortex
- IPAC:
-
Interstitial nucleus of the posterior limb of the anterior commissure
- JCMS:
-
Jaw-closing muscle spindle
- LGP:
-
Lateral globus pallidus
- LO:
-
Lateral orbital cortex
- MD:
-
Mediodorsal thalamic nucleus
- Me5:
-
Trigeminal mesencephalic nucleus
- ml:
-
Medial lemniscus
- MO:
-
Medial orbital cortex
- Mo5:
-
Trigeminal motor nucleus
- mt:
-
Mammillothalamic tract
- OPC:
-
Oval paracentral thalamic nucleus
- Or:
-
Orbital cortex
- Pb:
-
Parabrachial nucleus
- PB:
-
Phosphate buffer
- PBS:
-
Phosphate-buffered saline
- PC:
-
Paracentral thalamic nucleus
- PF:
-
Parafascicular thalamic nucleus
- Pom:
-
Medial part of posterior thalamic complex
- PrL:
-
Prelimbic cortex
- RF:
-
Rhinal fissure
- Rt:
-
Reticular thalamic nucleus
- S1:
-
Primary somatosensory cortex
- S2:
-
Secondary somatosensory cortex
- sm:
-
Stria medullaris of the thalamus
- Sm:
-
Submedial thalamic nucleus
- Sol:
-
Solitary tract nucleus
- Su5:
-
Supratrigeminal nucleus
- TSNC:
-
Trigeminal sensory nuclear complex
- Va:
-
Outer part of layer V
- VA:
-
Ventral anterior thalamic nucleus
- Vb:
-
Inner part of layer V
- VL:
-
Ventrolateral thalamic nucleus
- VM:
-
Ventromedial thalamic nucleus
- VO:
-
Ventral orbital cortex
- VPL:
-
Ventral posterolateral thalamic nucleus
- VPLo:
-
Oral region of the VPL
- VPM:
-
Ventral posteromedial thalamic nucleus
- VPMcvm:
-
Caudo-ventromedial edge of the VPM
- VPPC:
-
Parvicellular part of the ventral posterior thalamic nucleus
References
Akhter F, Haque T, Sato F, Kato T, Ohara H, Fujio T, Tsutsumi K, Uchino K, Ssssle BJ, Yoshida A (2014) Projections from the dorsal peduncular cortex to the trigeminal subnucleus caudalis (medullary dorsal horn) and other lower brainstem areas in rats. Neuroscience 266:23–37
Allen GV, Saper CB, Hurley KM, Cechetto DF (1991) Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol 311:1–16
Amassian VE, Berlin L (1958) Early cortical projection of group I afferents in forelimb muscle nerves of cat. J Physiol 143:61
Andersson SA, Landgren S, Wolsk D (1966) The thalamic relay and cortical projection of group I muscle afferents from the forelimb of the cat. J Physiol 183:576–591
Augustine JR (1985) The insular lobe in primates including humans. Neurol Res 7:2–10
Augustine JR (1996) Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22:229–244
Benison AM, Rector DM, Barth DS (2007) Hemispheric mapping of secondary somatosensory cortex in the rat. J Neurophysiol 97:200–207
Benjamin RM, Akert K (1959) Cortical and thalamic areas involved in taste discrimination in the albino rat. J Comp Neurol 111:231–259
Benjamin RM, Pfaffmann C (1955) Cortical localization of taste in albino rat. J Neurophysiol 18:56–64
Berendse HW, Groenewegen HJ (1991) Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience 42:73–102
Carvell GE, Simons DJ (1986) Somatotopic organization of the second somatosensory area (SII) in the cerebral cortex of the mouse. Somatosens Res 3:213–237
Cechetto DF, Saper CB (1987) Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol 262:27–45
Chang Z, Haque T, Iida C, Seki S, Sato F, Kato T, Uchino K, Ono T, Nakamura M, Bae YC, Yoshida A (2009) Distribution of premotoneurons for jaw-closing and jaw-opening motor nucleus receiving contacts from axon terminals of primary somatosensory cortical neurons in rats. Brain Res 1275:43–53
Coggeshall RE, Lekan HA (1996) Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol 364:6–15
Donoghue JP, Parham C (1983) Afferent connections of the lateral agranular field of the rat motor cortex. J Comp Neurol 217:390–404
Donoghue JP, Wise SP (1982) The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol 212:76–88
Dostrovsky JO, Guilbaud G (1988) Noxious stimuli excite neurons in nucleus submedius of the normal and arthritic rat. Brain Res 460:269–280
Dostrovsky JO, Broton JG, Warma NK (1987) Functional properties of subnucleus caudalis lamina I neurons projecting to nucleus submedius. In: Schmidt RF, Schaible H-G, Vahle-Hinz C (eds) Fine afferent nerve fibers and pain. Springer, Germany
Dubner R, Sessle BJ, Storey AT (1978) The neural basis of oral and facial function. Plenum Press, New York
Fang PC, Stepniewska I, Kaas JH (2006) The thalamic connections of motor, premotor, and prefrontal areas of cortex in a prosimian primate (Otolemur garnetti). Neuroscience 143:987–1020
Felleman DJ, Wall JT, Cusick CG, Kaas JH (1983) The representation of the body surface in S-I of cats. J Neurosci 3:1648–1669
Francis JT, Xu S, Chapin JK (2008) Proprioceptive and cutaneous representations in the rat ventral posterolateral thalamus. J Neurophysiol 99:2291–2304
Friedman DP, Jones EG (1981) Thalamic input to areas 3a and 2 in monkeys. J Neurophysiol 45:59–85
Fujio T, Sato F, Tachibana Y, Kato T, Tomita A, Higashiyama K, Ono T, Maeda Y, Yoshida A (2016) Revisiting the supratrigeminal nucleus in the rat. Neuroscience 324:307–320
Furuta T, Deschênes M, Kaneko T (2011) Anisotropic distribution of thalamocortical boutons in barrels. J Neurosci 31:6432–6439
Gandevia SC, Macefield G (1989) Projection of low-threshold afferents from human intercostal muscles to the cerebral cortex. Respir Physiol 77:203–214
Gauriau C, Bernard JF (2004) Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci 24:752–761
Groenewegen HJ, Galis-de Graaf Y, Smeets WJ (1999) Integration and segregation of limbic cortico-striatal loops at the thalamic level: an experimental tracing study in rats. J Chem Neuroanat 16:167–185
Hamilton RB, Norgren R (1984) Central projections of gustatory nerves in the rat. J Comp Neurol 222:560–577
Hanamori T, Kunitake T, Kato K, Kannan H (1998a) Neurons in the posterior insular cortex are responsive to gustatory stimulation of the pharyngolarynx, baroreceptor and chemoreceptor stimulation, and tail pinch in rats. Brain Res 785:97–106
Hanamori T, Kunitake T, Kato K, Kannan H (1998b) Responses of neurons in the insular cortex to gustatory, visceral, and nociceptive stimuli in rats. J Neurophysiol 79:2535–2545
Haque T, Yamamoto S, Masuda Y, Kato T, Sato F, Uchino K, Oka A, Nakamura M, Takeda R, Ono T, Kogo M, Yoshida A (2010) Thalamic afferent and efferent connectivity to cerebral cortical areas with direct projections to identified subgroups of trigeminal premotoneurons in the rat. Brain Res 1346:69–82
Haque T, Akhter F, Kato T, Sato F, Takeda R, Higashiyama K, Moritani M, Bae YC, Sessle BJ, Yoshida A (2012) Somatotopic direct projections from orofacial areas of secondary somatosensory cortex to trigeminal sensory nuclear complex in rats. Neuroscience 219:214–233
Haroian AJ, Massopust LC, Young PA (1981) Cerebellothalamic projections in the rat: an autoradiographic and degeneration study. J Comp Neurol 197:217–236
Heath CJ, Hore J, Phillips CG (1976) Inputs from low threshold muscle and cutaneous afferents of hand and forearm to areas 3a and 3b of baboon’s cerebral cortex. J Physiol (Lond) 257:199–227
Herbert H, Moga MM, Saper CB (1990) Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293:540–580
Herkenham M (1980) Laminar organization of thalamic projections to the rat neocortex. Science 207:532–535
Hicks RR, Huerta MF (1991) Differential thalamic connectivity of rostral and caudal parts of cortical area Fr2 in rats. Brain Res 568:325–329
Iida C, Oka A, Moritani M, Kato T, Haque T, Sato F, Nakamura M, Uchino K, Seki S, Bae YC, Takada K, Yoshida A (2010) Corticofugal direct projections to primary afferent neurons in the trigeminal mesencephalic nucleus of rats. Neuroscience 169:1739–1757
Ikeda T, Terayama R, Jue S-S, Sugiyo S, Dubner R, Ren K (2003) Differential rostral projections of caudal brainstem neurons receiving trigeminal input after masseter inflammation. J Comp Neurol 465:220–233
Ikenoue E, Akhter F, Tsutsumi Y, Sato F, Ohara H, Uchino K, Furuta T, Tachibana Y, Yoshida A (2018) Transcortical descending pathways through granular insular cortex conveying orofacial proprioception. Brain Res 1687:11–19
Ito S (1992) Multiple projection of vagal non-myelinated afferents to the anterior insular cortex in rats. Neurosci Lett 148:151–154
Iwai H, Kuramoto E, Yamanaka A, Sonomura T, Uemura M, Goto T (2015) Ascending parabrachio-thalamo-striatal pathways: potential circuits for integration of gustatory and oral motor functions. Neuroscience 294:1–13
Iwamura Y, Tanaka M, Sakamoto M, Hikosaka O (1983) Functional subdivisions representing different finger regions in area 3 of the first somatosensory cortex of the conscious monkey. Exp Brain Res 51:315–326
Iwata K, Itoga H, Ikukawa A, Hanashima N, Sumino R (1985) Distribution and response characteristics of masseteric nerve-driven neurons in two separate cortical projection areas of cats. Brain Res 342:179–182
Jones EG (2007) The Thalamus. Cambridge University Press, Cambridge
Jones EG, Friedman DP (1982) Projection pattern of functional components of thalamic ventrobasal complex on monkey somatosensory cortex. J Neurophysiol 48:521–544
Jones EG, Friedman DP, Hendry SH (1982) Thalamic basis of place- and modality-specific columns in monkey somatosensory cortex: a correlative anatomical and physiological study. J Neurophysiol 48:545–568
Kosar E, Grill HJ, Norgren R (1986) Gustatory cortex in the rat II Thalamocortical projections. Brain Res 379:342–352
Krettek JE, Price JL (1977a) The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 171:157–192
Krettek JE, Price JL (1977b) Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. J Comp Neurol 172:723–752
Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, Silver R (2004) Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J Comp Neurol 468:361–379
Lanciego LJ, Wouterlood FG (2011) A half century of experimental neuroanatomical tracing. Chem Neuroanat 42:157–383
Landgren S, Silfvenius H (1969) Projection to cerebral cortex of group I muscle afferents from the cat’s hind limb. J Physiol 200:353–372
Leak RK, Moore RY (2001) Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol 433:312–334
Leonard CM (1969) The prefrontal cortex of the rat. I. Cortical projection of the mediodorsal nucleus. II. Efferent connections. Brain Res 12:321–343
Lund JP, Sessle BJ (1974) Oral-facial and jaw muscle afferent projections to neurons in cat frontal cortex. Exp Neurol 45:314–331
Lund JP, Richmond FJ, Touloumis C, Patry Y, Lamarre Y (1978) The distribution of Golgi tendon organs and muscle spindles in masseter and temporalis muscles of the cat. Neuroscience 3:259–270
Macefield G, Burke D, Gandevia SC (1989) The cortical distribution of muscle and cutaneous afferent projections from the human foot. Electroencephalogr Clin Neurophysiol 72:518–528
Maeda N, Kobashi M, Mitoh Y, Fujita M, Minagi S, Matsuo R (2014) Differential involvement of two cortical masticatory areas in submandibular salivary secretion in rats. Brain Res 1543:200–208
Maendly R, Rüegg DG, Wiesendanger M, Wiesendanger R, Lagowska J, Hess B (1981) Thalamic relay for group I muscle afferents of forelimb nerves in the monkey. J Neurophysiol 46:901–917
Markowitsch HJ, Pritzel M (1981) Prefrontal cortex of the guinea pig (Cavia porcellus) defined as cortical projection area of the thalamic mediodorsal nucleus. Brain Behav Evol 18:80–95
Mesulam MM, Mufson EJ (1982) Insula of the old world monkey. III: efferent cortical output and comments on function. J Comp Neurol 212:38–52
Moga MM, Saper CB (1994) Neuropeptide-immunoreactive neurons projecting to the paraventricular hypothalamic nucleus in the rat. J Comp Neurol 346:137–150
Nakashima M, Uemura M, Yasui K, Ozaki HS, Tabata S, Taen A (2000) An anterograde and retrograde tract-tracing study on the projections from the thalamic gustatory area in the rat: distribution of neurons projecting to the insular cortex and amygdaloid complex. Neurosci Res 36:297–309
Ogawa H, Wang XD (2002) Neurons in the cortical taste area receive nociceptive inputs from the whole body as well as the oral cavity in the rat. Neurosci Lett 322:87–90
Ohno S, Kuramoto E, Furuta T, Hioki H, Tanaka YR, Fujiyama F, Sonomura T, Uemura M, Sugiyama K, Kaneko T (2012) A morphological analysis of thalamocortical axon fibers of rat posterior thalamic nuclei: a single neuron tracing study with viral vectors. Cereb Cortex 22:2840–2857
Oscarsson O, Rosén I (1963) Projection to cerebral cortex of large muscle-spindle afferents in forelimb nerves of the cat. J Physiol 169:924–945
Oscarsson O, Rosén I (1966) Short-latency projections to the cat’s cerebral cortex from skin and muscle afferents in the contralateral forelimb. J Physiol 182:164–184
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, Sydney
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, Sydney
Paxinos G, Watson C (2014) The rat brain in stereotaxic coordinates, 7th edn. Academic Press, Sydney
Phillips CG, Powell TP, Wiesendanger M (1971) Projection from low-threshold muscle afferents of hand and forearm to area 3a of baboon’s cortex. J Physiol 217:419–446
Pierret T, Lavallée P, Deschênes M (2000) Parallel streams for the relay of vibrissal information through thalamic barreloids. J Neurosci 20:7455–7462
Powell TP, Mountcastle VB (1959) Some aspects of the functional organization of the cortex of the postcentral gyrus of the monkey: a correlation of findings obtained in a single unit analysis with cytoarchitecture. Bull Johns Hopkins Hosp 105:133–162
Sadikot AF, Parent A, François C (1992) Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J Comp Neurol 315:137–159
Sato F, Akhter F, Haque T, Kato T, Takeda R, Nagase Y, Sessle BJ, Yoshida A (2013) Projections from the insular cortex to pain-receptive trigeminal caudal subnucleus (medullary dorsal horn) and other lower brainstem areas in rats. Neuroscience 233:9–27
Sato F, Uemura Y, Kanno C, Tsutsumi Y, Tomita A, Oka A, Kato T, Uchino K, Murakami J, Haque T, Tachibana Y, Yoshida A (2017) Thalamo-insular pathway conveying orofacial muscle proprioception in the rat. Neuroscience 365:158–178
Sato F, Kado S, Tsutsumi Y, Tachibana Y, Ikenoue E, Furuta T, Uchino K, Bae YC, Uzawa N, Yoshida A (2020) Ascending projection of jaw-closing muscle-proprioception to the intralaminar thalamic nuclei in rats. Brain Res 1739:146830
Satoh Y, Ishizuka K, Murakami T (2007) Changes in cortically induced rhythmic jaw movements after lesioning of the red nucleus in rats. Brain Res 1165:60–70
Sirisko MA, Sessle BJ (1983) Corticobulbar projections and orofacial and muscle afferent inputs of neurons in primate sensorimotor cerebral cortex. Exp Neurol 82:716–720
Stepniewska I, Preuss TM, Kaas JH (2007) Thalamic connections of the dorsal and ventral premotor areas in New World owl monkeys. Neuroscience 147:727–745
Tomita A, Kato T, Sato F, Haque T, Oka A, Yamamoto M, Ono T, Bae YC, Maeda Y, Sessle BJ, Yoshida A (2012) Somatotopic direct projections from orofacial areas of primary somatosensory cortex to pons and medulla, especially to trigeminal sensory nuclear complex, in rats. Neuroscience 200:166–185
Tsutsumi Y, Tachibana Y, Sato F, Furuta T, Ohara H, Tomita A, Fujita M, Moritani M, Yoshida A (2018) Cortical and subcortical projections from granular insular cortex receiving orofacial proprioception. Neuroscience 388:317–329
Uemura Y, Haque T, Sato F, Tsutsumi Y, Ohara H, Oka A, Furuta T, Bae YC, Yamashiro T, Tachibana Y, Yoshida A (2020) Proprioceptive thalamus receiving forelimb and neck muscle spindle inputs via the external cuneate nucleus in the rat. Brain Struct Funct 225:2177–2192
Van der Werf YD, Witter MP, Groenewegen HJ (2002) The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev 39:107–140
Van Eden CG, Lamme VA, Uylings HB (1992) Heterotopic cortical afferents to the medial prefrontal cortex in the rat. A combined retrograde and anterograde tracer study. Eur J Neurosci 4:77–97
Waite PME (2004) Trigeminal sensory system. In: Paxinos G (ed) The rat nervous system, 3rd edn. Elsevier Academic Press, San Diego, pp 817–851
Waite PME, Tracey DJ (1985) Trigeminal sensory system. In: Paxinos G (ed) The rat nervous system, 2nd edn. Academic Press, San Diego, pp 705–724
Welker C, Sinha MM (1972) Somatotopic organization of Sm II cerebral neocortex in albino rat. Brain Res 37:132–136
Wouterlood FG (2015) A survey of current Neuroanatomical tracing techniques. In: Arenkiel BR (ed) Neural tracing methods. Humana Press, New York, Tracing neurons and their connections, pp 1–49
Yamamoto T, Yuyama N, Kawamura Y (1981) Cortical neurons responding to tactile, thermal and taste stimulations of the rat’s tongue. Brain Res 221:202–206
Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R (1988) Sensory inputs from the oral region to the cerebral cortex in behaving rats: an analysis of unit responses in cortical somatosensory and taste areas during ingestive behavior. J Neurophysiol 60:1303–1321
Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R (1989) Sensory and motor responses of trigeminal and reticular neurons during ingestive behavior in rats. Exp Brain Res 76:386–400
Yoshida A, Dostrovsky JO, Sessle BJ, Chiang CY (1991) Trigeminal projections to the nucleus submedius of the thalamus in the rat. J Comp Neurol 307:609–625
Yoshida A, Dostrovsky JO, Chiang CY (1992) The afferent and efferent connections of the nucleus submedius in the rat. J Comp Neurol 324:115–133
Yoshida A, Fukami H, Nagase Y, Appenteng K, Honma S, Zhang LF, Bae YC, Shigenaga Y (2001) Quantitative analysis of synaptic contacts made between functionally identified oralis neurons and trigeminal motoneurons in cats. J Neurosci 21:6298–6307
Yoshida A, Yamamoto M, Moritani M, Fukami H, Bae YC, Chang Z, Sugiyo S, Takemura M, Park KP, Shigenaga Y (2005) Bilateral projection of functionally characterized trigeminal oralis neurons to trigeminal motoneurons in cats. Brain Res 1036:208–212
Yoshida A, Taki I, Chang Z, Iida C, Haque T, Tomita A, Seki S, Yamamoto S, Masuda Y, Moritani M, Shigenaga Y (2009) Corticofugal projections to trigeminal motoneurons innervating antagonistic jaw muscles in rats as demonstrated by anterograde and retrograde tract-tracing. J Comp Neurol 514:368–386
Yoshida A, Fujio T, Sato F, Ali MS, Haque T, Ohara H, Moritani M, Kato T, Dostrovsky JO, Tachibana Y (2017) Orofacial proprioceptive thalamus of the rat. Brain Struct Funct 222:2655–2669
Zhang ZW, Deschênes M (1998) Projections to layer VI of the posteromedial barrel field in the rat: a reappraisal of the role of corticothalamic pathways. Cereb Cortex 8:428–436
Zhang GX, Sasamoto K (1990) Projections of two separate cortical areas for rhythmical jaw movements in the rat. Brain Res Bull 24:221–230
Acknowledgements
We are indebted to Dr. Katsuro Uchino, Dr. Haruka Ohara, and Dr. Yume Uemura for their technical help. This work was supported by Grants-in-Aid for Scientific Research of the Japan Society for the Promotion of Science (18K19641, 18KK0259 to A.Y. and 17K11608, 20K09888 to F.S.).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. AY and YTa conceptualized the hypothesis, designed and supervised the experiments and directed the data analysis. YTs, YM, TH, and FS carried out the experiments and data analysis. TF and AO helped perform experiments and data analysis. AY, YTs, TF, MM, YCB, TY, and YTa finalized the figures and text.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsutsumi, Y., Mizuno, Y., Haque, T. et al. Widespread corticopetal projections from the oval paracentral nucleus of the intralaminar thalamic nuclei conveying orofacial proprioception in rats. Brain Struct Funct 226, 1115–1133 (2021). https://doi.org/10.1007/s00429-021-02228-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-021-02228-5