Summary
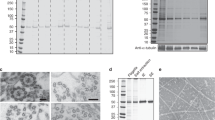
The nutritive tubes of telotrophic insect ovaries are cytoplasmic channels along which ribosomes are transported over distances of several mm from trophic cells to the developing oocytes. The presence within the nutritive tubes of a massive number of orientated microtubules renders them strongly birefringent in polarised light, a property which, together with their size, rendered them amenable to isolation by microdissection. Ultrastructurally the isolated tubes were indistinguishable from undissected controls. Polyacrylamide gels revealed a consistent pattern of some 30 bands of which tubulin was the most prominent. The tubes also contained a band which comigrated with the major high molecular weight micro tubule associated protein (MAP) from mouse brain but no detectable actin, myosin or dynein. Microtubules in the isolated tubes were not depolymerised by treatments (cold, calcium and colchicine) which typically disrupt cytoplasmic microtubules. Following extraction of the membrane enclosing the tubes and the cytoplasmic matrix the microtubule cytoskeleton persisted, retaining its cylindrical organisation although no bridges between the microtubules were detected in the electron microscope. The possibility that the stability and spatial deployment of the nutritive tube microtubules is conferred by specific microtubule accessory proteins is discussed.
Similar content being viewed by others
References
Borisy, G.G., Olmsted, J.B.: Nucleated assembly of microtubules in porcine brain extracts. Science 177, 1196–1197 (1972)
Dentler, W.L., Granett, S., Rosenbaum, J.L.: Ultrastructural localization of the high molecular weight proteins associated with in vitro assembled microtubules. J. Cell Biol. 65, 237–241 (1975)
Edds, K.T.: Motility in Echinosphaerum nucleofilum. I. An analysis of particle motions in the axopodia and a direct test of the involvement of the axoneme. J. Cell Biol. 66, 145–155 (1975a)
Edds, K.T.: Motility in Echinosphaerum nucleofilum. II. Cytoplasmic contractility and its molecular basis. J. Cell Biol. 66, 155–164 (1975b)
Fairbanks, G., Steck, T.L., Wallach, D.F.H.: Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10, 2606–2617 (1971)
Farrell, K.W., Wilson, L.: Microtubule reassembly in vitro with tubulin from outer doublet microtubules. J. Cell Biol. 75, 272a (1977)
Gibbons, I.R.: Chemical dissection of cilia. Arch. Biol. (Liège) 76, 317–352 (1965)
Good, N.E., Winget, G.D., Winter, N., Connolly, T.N., Izawa, S., Singh, R.M.M.: Hydrogen ion buffers for biological research. Biochemistry 5, 467–477 (1966)
Hyams, J.S., Stebbings, H.: The distribution and function of microtubules in nutritive tubes. Tissue and Cell 9, 537–545 (1977a)
Hyams, J.S., Stebbings, H.: The isolation and characterisation of a cytoplasmic microtubule transport system. J. Cell Biol. 75, 271a (1977b)
Hyams, J.S., Stebbings, H.: Microtubule associated cytoplasmic transport. In: Microtubules, (K. Roberts and J.S. Hyams, Eds.) New York-London: Academic Press, in press 1979
Inoué, S., Sato, H.: Cell motility by labile association of molecules. J. Gen. Physiol. 50, 259–292 (1967)
Kaulenas, M.S., Bosselman, R.A.: Functional and structural differences in ribosomal subunits isolated from monomers and polysomes of Acheta domesticus. Comp. Biochem. Physiol [B] 49, 415–430 (1974)
Kuriyama, R.: In vitro polymerization of flagellar and ciliary outer fiber tubulin into microtubules. J. Biochem. (Tokyo) 80, 153–165 (1976)
Linck, R.W.: Comparative isolation of cilia and flagella from the lamellibranch mollusc, Aequipecten irradians. J. Cell Sci. 12, 345–367 (1973)
Luftig, R.B., McMillan, P.N., Weatherbee, J.A., Weihing, R.R.: Increased visualization of microtubules by an improved fixation procedure. J. Histochem. Cytochem. 25, 175–187 (1977)
Macgregor, H.C., Stebbings, H.: A massive system of microtubules associated with cytoplasmic movement in telotrophic ovarioles. J. Cell Sci. 6, 431–449 (1970)
Murphy, D.B., Borisy, G.G.: Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc. Nat. Acad. Sci. USA 72, 2696–2700 (1975)
Murphy, D.B., Johnson, K.A., Borisy, G.G.: Role of tubulin-associated proteins in microtubule nucleation and elongation. J. Mol. Biol. 117, 33–52 (1977)
Murphy, D.B., Tilney, L.G.: The role of microtubules in the movement of pigment granules in teleost melanophores. J. Cell Biol. 61, 757–779 (1974)
Payne, J.W.: Polymerization of proteins with glutaraldehyde. Soluble molecular-weight markers. Biochem. J. 135, 867–875 (1973)
Rebhun, L.I.: Polarized intracellular particle transport: saltatory movements and cytoplasmic streaming. Int. Rev. Cytol. 32, 93–137 (1972)
Shapiro, A.L., Vinuela, E., Maizel, J.V.: Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem. Biophys. Res. Commun. 28, 815–820 (1967)
Smith, D.S.: On the significance of cross-bridges between microtubules and synaptic vesicles. Philos. Trans. R. Soc. Lond. [Biol.] 261, 395–405 (1971)
Stebbings, H., Bennett, C.E.: The effect of colchicine on the sleeve element of microtubules. Exp. Cell Res. 100, 419–423 (1976)
Stephens, R.E.: High resolution preparative SDS-polyacrylamide gel electrophoresis: fluorescent visualization and electrophoretic elution-concentration of protein bands. Anal. Biochem. 65, 369–379 (1975)
Summers, K.E., Gibbons, I.R.: Effects of trypsin digestion on flagellar structures and their relationship to motility. J. Cell Biol. 58, 618–629 (1973)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hyams, J.S., Stebbings, H. The mechanism of microtubule associated cytoplasmic transport. Cell Tissue Res. 196, 103–116 (1979). https://doi.org/10.1007/BF00236351
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00236351