Summary
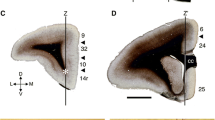
Large neurons of the mouse caudate nucleus contain profuse Nissl material, have a maximal pole-to-pole diameter of up to 25 μm, occur preferentially within a central or CORE zone of the neostriatum, and are almost always located within territories delimited by medium-sized clustered neurons. Examination of coronal sections taken through the head of the nucleus and stained with cresyl violet revealed the absence of age-related pathology as evaluated by three parameters: (1) there was no statistically significant difference in the frequency of large neurons in all areas of the head of the nucleus; (2) the large neuron continued to be confined preferentially to the CORE zone of the head of the nucleus throughout the time period studied; and (3) no statistically significant shifts were detected in the geometry of large cell-medium cell clusters.
Since postnatal age did not significantly affect the frequency, distribution or geometry of large cell-medium cell clusters, data from all animals was combined. This information was used to evaluate the possibility of an interaction between location within or outside the CORE zone and type of large cell-medium cell cluster, but these results were not statistically significant. Therefore, the data analyzed in this study support the view that the geometry of large cell-medium cell clusters is extremely stable in the mouse neostriatum and seems not to be influenced either by age of the animal or by location of the cell grouping.
Similar content being viewed by others
References
Ball MJ (1977) Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia. Acta Neuropathol (Berl) 37: 111–118
Bondareff W (1980) Synaptic organization as a function of aging. In: Adelman R, et al. (eds) Neural regulatory mechanisms during aging. Liss, New York, pp 143–158
Brizzee KR (1968) A comparison of cell populations at various depth levels in cerebral cortex of young adult and aged Long-Evans rats. J Gerontol 23: 289–297
Brizzee KR (1973) Quantitative histological studies on aging changes in cerebral cortex of Rhesus monkey and albino rat with notes on effects of prolonged low-dose ionizing irradiation in the rat. Prog Brain Res 40: 141–160
Brody H (1955) Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol 102: 511–526
Buell SJ, Coleman PD (1979) Dendritic growth in the aged human brain and failure of growth in senile dementia. Science 206: 854–856
Bugiani O, Salvarani S, Perdelli F, Mancardi GL, Leonardi A (1978) Nerve cell loss with aging in the putamen. Eur Neurol 14: 286–291
Chaconas G, Finch CE (1973) The effect of ageing on RNA/DNA ratios in brain regions of the C57B1/6J male mouse. J Neurochem 21: 1469–1473
Cupp CJ, Uemura E (1980) Age-related changes in prefrontal cortex of Macaca mulatta: Quantitative analysis of dendritic branching patterns. Exp Neurol 69: 143–163
Diamond MC, Johnson RE, Gold MW (1977) Changes in neuron number and size and glia number in the young, adult and aging rat medial occipital cortex. Behav Biol 20: 409–418
Finch CE (1973) Catecholamine metabolism in the brains of aging male mice. Brain Res 52: 261–276
Freund G (1980) Cholinergic receptor loss in brains of aging mice. Life Sci 26: 371–375
Geinisman Y (1981) Loss of axon terminals contacting neuronal somata in the dentate gyrus of aged rats. Brain Res 212: 136–139
Geinisman Y, Bondareff W, Dodge JT (1978) Hypertrophy of astroglial processes in the dentate gyrus of the senescent rat. Am J Anat 153: 537–544
Gibson GE, Peterson C, Jenden DJ (1981) Brain acetylcholine synthesis declines with senescence. Science 213: 674–676
Goldman-Rakic PS (1981) Prenatal formation of cortical input and development of cytoarchitectonic compartments in the neostriatum of the rhesus monkey. J Neurosci 1: 721–735
Landfield PW, Rose G, Sandles L, Wohlstadter TC, Lynch G (1977) Patterns of astroglial hypertrophy and neuronal degeneration in the hippocampus of aged memory-deficient rats. J Gerontol 149: 73–82
Makman MH, Ahn HS, Thal LJ, Sharpless NS, Dvorkin B, Horowitz SG, Rosenfeld M (1980) Evidence for selective loss of brain dopamine- and histamine-stimulated adenylate cyclase activities in rabbits with aging. Brain Res 192: 177–183
McGeer PL, McGeer EG, Suzuki JS (1977) Aging and extrapyramidal function. Arch Neurol 34: 33–35
Mensah PL (1977) The internal organization of the mouse caudate nucleus: Evidence for cell clustering and regional variation. Brain Res 137: 53–66
Mensah PL (1980) Distribution of the largest neuron in mouse caudate-putamen nucleus: Its position in large cell-medium cell clusters. Exp Brain Res 38: 267–271
Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB (1975) Progressive dendritic changes in aging human cortex. Exp Neurol 47: 392–403
Scheibel ME, Tomiyasu U, Scheibel AB (1977) The aging human Betz cell. Exp Neurol 56: 598–609
Severson JA, Finch CE (1980) Reduced dopaminergic binding during aging in the rodent striatum. Brain Res 192: 147–162
Sidman RL, Angevine JB Jr, Pierce ET (1971) Atlas of the mouse brain and spinal cord. Harvard University Press, Cambridge, Massachusetts
Sokal RR, Rohlf FJ (1969) Biometry. Freeman, San Francisco
Sturrock RR (1979) A quantitative lifespan study of changes in cell number, cell division and cell death in various regions of the mouse forebrain. Neuropathol Appl Neurobiol 5: 433–456
Sturrock RR (1980) A comparative quantitative and morphological study of ageing in the mouse neostriatum, indusium griseum and anterior commissure. Neuropathol Appl Neurobiol 6: 51–68
Thal LJ, Horowitz SG, Dvorkin B, Makman MH (1980) Evidence for loss of brain [3H] Spiroperidol and [3H] ADTN binding sites in rabbit brain with aging. Brain Res 192: 185–194
Uemura E (1980) Age-related changes in prefrontal cortex of Macaca mulatta: Synaptic density. Exp Neurol 69: 164–172
Vijayashankar N, Brody H (1977) Quantitative study of the pigmented neurons in the nuclei locus coeruleus and subcoeruleus in man as related to aging. J Neuropathol Exp Neurol 38: 490–497
Wree A, Braak H, Schleicher A, Zilles K (1980) Biomathematical analysis of the neuronal loss in the aging human brain of both sexes, demonstrated in pigment preparations of the pars cerebellaris loci coerulei. Anat Embryol (Berl) 160: 105–119
Author information
Authors and Affiliations
Additional information
Supported by a Biomedical Research Support Grant (NIH 5-SO7-RRO5356) to the University of Southern California School of Medicine and by a grant from the Neurosciences Institute, Los Angeles, CA
Rights and permissions
About this article
Cite this article
Mensah, P.L. Stability of large cell-medium cell clusters in the mature neostriatum. Exp Brain Res 47, 57–60 (1982). https://doi.org/10.1007/BF00235886
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00235886