Summary
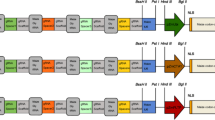
Cruciferin is the major seed storage protein in Brassica napus. As much as 1.9 kbp of the BnC1 cruciferin gene promoter have been sequenced and analyzed. Promoter fragments with 5′ deletions from −2500 to −v202 were fused with the ß-glucuronidase reporter gene and used for Nicotiana tabacum transformation. ß-glucuronidase could be specifically expressed in transgenic tobacco seeds under the control of the BnC1 promoter and regulatory elements were found to be dispersed over 1903 bp. An almost 5-fold increase in ß-glucuronidase expression was obtained when the promoter length was increased from −379 to −498, and another 10-fold increase was observed when sequences between −1266 and −1903 were added. Histochemical analysis shows that the region between −844 and −1266 directs the expression of the chimeric gene specifically to the root apical meristem.
Similar content being viewed by others
Abbreviations
- GUS:
-
ß-glucuronidase
- MU:
-
4-methyl umbelliferone
- MUG:
-
4-methyl-umbelliferyl-ß-D-glucuronide
- X-gluc:
-
5-bromo-4-chloro-3-indolyl-ß-D-glucuronide
References
Allen RD, Bernier F, Lessard RA, Beachy RN (1989) Nuclear factors interact with a soybean ß-conglycinin enhancer. Plant Cell 1:623–631
An G, Ebert PR, Mitra A, Ha SB (1988) Binary vectors. In: Gelvin SB, Schilperoort RA, Verma DPS (eds) Plant Molecular Biology Manual, Volume A3. Klumer Academic Publishers, Dordrecht, pp. 1–19
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 78:248–254
Breen JP, Crouch ML (1992) Molecular analysis of a cruciferin storage protein gene family of Brassica napus. Plant Mol Biol 19:1049–1055
Bustos MM, Guiltinan MJ, Jordano J, Begum D, Kalkan FA, Hall TC (1989) Regulation of ß-glucuronidase expression in transgenic tobacco plants by an A/Trich, cis-acting sequence found upstream of a French bean ß-phaseolin gene. Plant Cell 1:839–853
Chen Z-L, Schuler MA, Beachy RN (1986) Functional analysis of regulatory elements in a plant embryo-specific gene. Proc Natl Acad Sci USA 83:8560–8564
Crouch ML, Sussex IM (1981) Development and storage-protein synthesis in Brassica napus L. embryos in vivo and in vitro. Planta 153:64–74
DeLisle AJ, Crouch ML (1989) Seed storage protein transcription and mRNA levels in Brassica napus during development and in response to exogenous abscisic acid. Plant Physiol 91:617–623.
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12:387–395
Dickinson CD, Evans RP, Nielsen NC (1988) RY repeats are conserved in the 5′-flanking regions of legume seed-protein genes. Nucleic Acids Res 16:371
Ericson ML, Murén E, Gustavsson H-O, Josefsson L-G, Rask L (1991) Analysis of the promoter region of napin genes from Brassica napus demonstrates binding of nuclear protein in vitro to a conserved sequence motif. Eur J Biochem 197:741–746
Fernández DE, Turner FR, Crouch ML (1991) In situ localization of storage protein mRNAs in developing meristems of Brassica napus embryos. Development111:299–313
Finkelstein RR, Tenbarge KM, Shumway JE, Crouch ML (1985) Role of ABA in maturation of rapeseed embryos. Plant Physiol 78:630–636
García Blanco MA, Clerc RG, Sharp PA (1989) The DNA-binding homeodomain of the Oct-2 protein. Genes Dev 3:739–745
Henikoff S (1984) Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene 28:351–359
Höglund A-S, Rödin J, Larsson E, Rask L (1992) Distribution of napin and cruciferin in developing rape seed embryos. Plant Physiol 98:509–515
Hones NC, Rigby PWJ, Ziff EB (1988) Trans-acting factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev 2, 267–281
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Howell DC (1992) Statistical Methods for Psychology, Third Edition, PWS-Kent, Boston
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Reporter 5:387–405
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusion: ß-glucuronidase as a sensitive and versatile gene fusion marker in higher plant. EMBO J 6:3901–3907
Jofuku KD, Okamuro JK, Goldberg RB (1987) Interaction of an embryo DNA-binding protein with a soybean lectin gene upstream region. Nature328:734–737
Jordano J, Almoguera C, Thomas TL (1989) A sunflower helianthinin gene upstream sequence ensemble contains an enhancer and sites of nuclear protein interaction. Plant Cell 1:855–866
Lessard PA, Allen RD, Bernier F, Crispino JD, Fujiwara T, Beachy RN (1991) Multiple nuclear factors interact with upstream sequences of differentially regulated ß-conglycinin genes. Plant Mol Biol 16, 397–413
Maier U-G, Brown JWS, Toloczyki C, Feix G (1987) Binding of a nuclear factor to a consensus sequence in the 5′ flanking region of zein genes from maize. EMBO J 6:17–22
Marck C (1988) “DNA Strider”: a “C” program for the fast analysis of DNA and Protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res 16:1829–1836
Pang PP, Pruitt RE, Meyerowitz EM (1991) Molecular cloning, genomic organization, expression and evolution of 12S seed storage protein of Arabidopsis thaliana. Plant Mol Biol 11:805–820
Peach C, Velten J (1991) Transgene expression variability (position effect) of CAT and GUS reporter genes driven by linked divergent T-DNA promoters. Plant Mol Biol 17:49–60
Riggs CC, Voelker TA, Chrispeels MJD.(1989) Cotyledon nuclear proteins bind to DNA fragments harboring regulatory elements of phytohemagglutinin genes. Plant Cell 1:609–621
Rödin J, Ericson ML, Josefsson LG, Rask L (1990) Characterization of a cDNA clone encoding a Brassica napus 12S protein (cruciferin) subunit: Relationship between precursors and mature chains. J. Biol Chem 265:2720–2723
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Shirsat AH, Wilford N, Croy RRD (1989) Gene copy number and levels of expression in transgenic plants of a seed specific gene in tobacco seed. Plant Sci 61:75–80
Sjodahl S, Rödin J, Rask L (1991) Characterization of the 12S globulin complex of B. napus. Evolutionary relationship to other 11–12S storage globulins. Eur J Biochem 196:617–621
Sjödahl S, Gustavsson H-O, Rödin J, Lenman M, Höglund A-S, Rask L (1993) Cruciferin gene families are expressed coordinately but with tissue-specific differences during Brassica napus seed development. Plant Mol Biol 23:1165–1176
Takaiwa F, Oono K, Kato A (1991) Analysis of the 5′ flanking region responsible for the endosperm-specific expression of a rice glutelin chimeric gene in transgenic tobacco. Plant Mol Biol 16:49–58
Wilen RW, Mandel RM, Pharis RP, Holbrook LA, Moloney MM (1990) Effects of abscisic acid and high osmoticum on storage protein gene expression in microspore embryos of Brassica napus. Plant Physiol 94:875–881
Wilen RW, van Rooijen GJH, Pearce DW, Pharis RP, Holbrook LA, Moloney MM (1991) Effects of jasmonic acid on embryospecific processes in Brassica and Linum oilseeds. Plant Physiol 95:399–405
Author information
Authors and Affiliations
Additional information
Communicated by R. N. Beachy
Rights and permissions
About this article
Cite this article
Bilodeau, P., Lafontaine, JG. & Bellemare, G. Far upstream activating promoter regions are responsible for expression of the BnC1 cruciferin gene from Brassica napus . Plant Cell Reports 14, 125–130 (1994). https://doi.org/10.1007/BF00233775
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00233775